Isolation, Structural Analyses and Biological Activity Assays against Chronic Lymphocytic Leukemia of Two Novel Cytochalasins — Sclerotionigrin A and B
Abstract
:1. Introduction
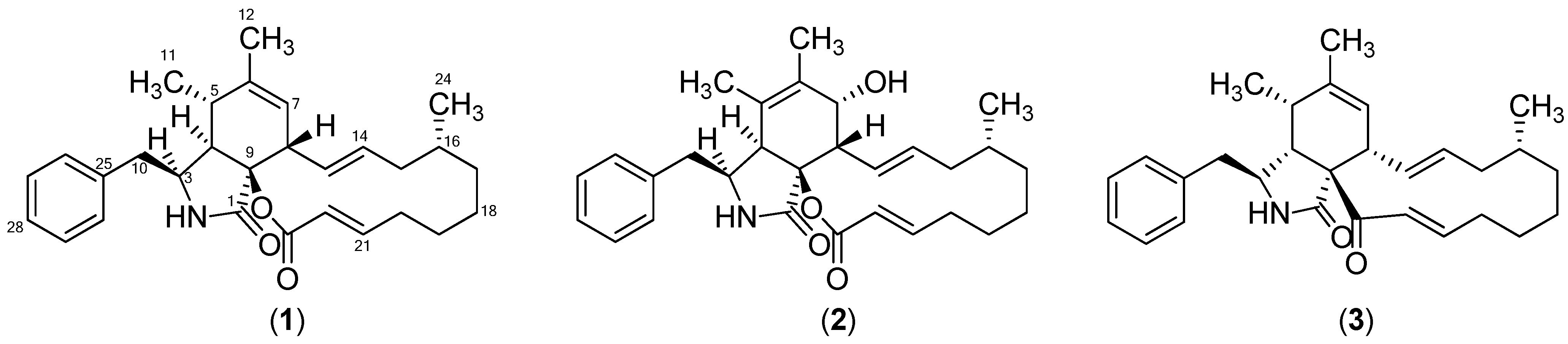
2. Results and Discussion
| No. | δH (Integral, Mult., J [Hz]) | δC | HMBC | NOESY |
|---|---|---|---|---|
| 1 | - | 170.6 | - | - |
| 2 | 8.00 (1H, s) | - | 1, 3, 4, 9 | 3, 10 |
| 3 | 3.09 (1H, td, 5.8, 3.1) | 54.2 | - | 2, 4, 10, 11, 12, 26/30 |
| 4 | 2.53 (1H, dd, 4.2, 3.1) | 49.1 | 3, 5, 6, 9 | 3, 10, 11, 26/30 |
| 5 | 2.57 (1H, m) | 33.6 | - | 7, 8, 11 |
| 6 | - | 140.0 | - | - |
| 7 | 5.25 (1H, m) | 123.6 | - | 5, 8, 12 |
| 8 | 3.15 (1H, m) | 45.8 | - | 5, 7, 13, 14 |
| 9 | - | 85.4 | - | - |
| 10 | 2.82 (2H, m) | 42.6 | 3, 4, 25, 26/30 | 3, 4, 26/30 |
| 11 | 0.68 (3H, d, 7.1) | 12.8 | 4, 5, 6 | 3, 4, 5, 12, 26/30 |
| 12 | 1.65 (3H, s) | 19.4 | 5, 6, 7 | 3, 7, 11 |
| 13 | 5.85 (1H, ddd, 14.8, 10.0, 1.2) | 128.9 | 15 | 8, 15 |
| 14 | 5.22 (1H, m) | 132.6 | 8 | 8, 15', 16 |
| 15 | 1.60 (1H, d, 13.5) | 40.7 | 13, 14, 16 | 13, 15' |
| 15' | 2.07 (1H, dd, 13.5, 2.1) | 40.7 | - | 14, 15, 16, 20', 24 |
| 16 | 1.36 (1H, m) | 31.9 | - | 14, 15', 19' |
| 17 | 0.61 (1H, m) | 33.8 | - | 17', 18', 24 |
| 17' | 1.66 (1H, m) | 33.8 | - | 17 |
| 18 | 1.14 (1H, m) | 25.9 | - | 18' |
| 18' | 1.53 (1H, m) | 25.9 | - | 17, 18 |
| 19 | 1.29 (1H, m) | 25.3 | - | 24 |
| 19' | 1.68 (1H, m) | 25.3 | - | 16, 21 |
| 20 | 2.23 (1H, m) | 33.1 | - | 20', 21 |
| 20' | 2.29 (1H, m) | 33.1 | - | 15', 20, 22 |
| 21 | 6.96 (1H, ddd, 15.5, 8.6, 6.8) | 151.7 | 23 | 19', 20, 22 |
| 22 | 5.65 (1H, d, 15.5) | 120.6 | 20, 23 | 20', 21 |
| 23 | - | 163.5 | - | - |
| 24 | 0.84 (3H, d, 6.3) | 20.0 | 15, 16, 17 | 15', 17, 19 |
| 25 | - | 137.8 | - | - |
| 26 ‡ | 7.14 (1H, app. d, 7.5) | 129.5 | 10, 26/30, 28 | 3, 4, 10, 11 |
| 27 ‡ | 7.26 (1H, app. t, 7.4) | 128.0 | 25, 29 | - |
| 28 | 7.18 (1H, app. t, 7.5) | 126.1 | 26, 30 | - |
| 29 ‡ | 7.26 (1H, app. t, 7.5) | 128.0 | 25, 27 | - |
| 30 ‡ | 7.14 (1H, app. d, 7.5) | 129.5 | 10, 26/30, 28 | 3, 4, 10, 11 |

| No. | δH (Integral, Mult., J [Hz]) | δC | HMBC | NOESY |
|---|---|---|---|---|
| 1 | - | 171.2 | - | - |
| 2 | 8.34 (1H, br. s) | - | 3, 4, 9 | 3, 10' |
| 3 | 3.39 (1H, m) | 57.7 | 1, 4, 5, 9 | 2, 10, 10', 11, 26/30 |
| 4 | 3.32 (1H, m) | 47.1 | 1, 5, 6, 9 | 13, 26/30 |
| 5 | - | 123.9 | - | - |
| 6 | - | 134.2 | - | - |
| 7 | 3.68 (1H, d, 9.7) | 69.1 | - | 8, 12, 13 |
| 8 | 3.05 (1H, t, 10.0) | 48.3 | 1, 4, 7, 9, 13, 14 | 7, 13, 14 |
| 9 | - | 83.6 | - | - |
| 10 | 2.55 (1H, dd, 13.0, 10.1) | 42.5 | 3, 4, 25, 26/30 | 3, 10', 26/30 |
| 10' | 2.92 (1H, dd, 13.0, 5.0) | 42.5 | 3, 4, 25, 26/30 | 2, 3, 10, 26/30 |
| 11 | 1.16 (3H, s) | 16.7 | 4, 5, 6 | 3, 26/30 |
| 12 | 1.52 (3H, s) | 14.3 | 5, 6, 7 | 7 |
| 13 | 6.03 (1H, dd, 15.0, 11.3) | 128.4 | 8, 15/15’ | 4, 7, 8, 14, 15 |
| 14 | 5.00 (1H, ddd, 15.0, 10.8, 3.4) | 132.7 | 8, 15/15’ | 8, 13, 15, 15' |
| 15 | 1.58 (1H, dt, 13.0, 11.1) | 41.6 | 16 | 13, 14, 15', 16, 17' |
| 15' | 2.00 (1H, m) | 41.6 | - | 14, 15, 16, 24 |
| 16 | 1.13 (1H, m) | 32.5 | - | 15, 15', 17', 18, 24 |
| 17 | 0.52 (1H, m) | 34.5 | - | 17' |
| 17' | 1.67 (1H, m) | 34.5 | 24 | 15, 16, 17, 18, 24 |
| 18 | 0.86 (1H, m) | 26.1 | - | 16, 17', 18' |
| 18' | 1.68 (1H, m) | 26.1 | 20 | 18, 19, 21 |
| 19 | 1.30 (1H, m) | 25.4 | - | 18', 19' |
| 19' | 1.73 (1H, m) | 25.4 | - | 19 |
| 20 | 2.11 (1H, m) | 33.4 | - | 20', 22 |
| 20' | 2.41 (1H, m) | 33.4 | - | 20, 21 |
| 21 | 6.89 (1H, ddd, 15.7, 10.8, 5.0) | 151.4 | 20, 23 | 18', 20', 22 |
| 22 | 5.79 (1H, d, 16.1) | 121.3 | 20, 23 | 20, 21 |
| 23 | - | 163.8 | - | - |
| 24 | 0.83 (3H, d, 6.6) | 19.9 | 15, 16, 17 | 15', 16, 17' |
| 25 | - | 137.4 | - | - |
| 26 ‡ | 7.08 (1H, app. d, 7.1) | 128.9 | 10, 28, 30 | 3, 4, 10, 10', 11, 27/29 |
| 27 ‡ | 7.31 (1H, app. t, 7.5) | 128.2 | 25, 29 | 26/30, 28 |
| 28 | 7.23 (1H, app. t, 7.4) | 126.3 | 26, 30 | 27/29 |
| 29 ‡ | 7.31(1H, app. t, 7.5) | 128.2 | 25, 27 | 26/30, 28 |
| 30 ‡ | 7.08 (1H, app. d, 7.1) | 128.9 | 10, 26, 28 | 3, 4, 10, 10',11, 27/29 |

| Compound | CLL | Healthy B-Cells |
|---|---|---|
| Sclerotionigrin A (1) | 72 µM | No effect |
| Sclerotionigrin B (2) | No effect | No effect |
| Proxiphomin (3) | 48 µM | No effect |
3. Experimental Section
3.1. Fungal Growth and Extraction
3.2. Preparative Isolation of Cytochalasins
3.3. Chemical Analysis
3.4. NMR and Optical Roation
3.5. CLL Cells, Cell Viability and Apoptosis Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zenz, T.; Mertens, D.; Küppers, R.; Döhner, H.; Stilgenbauer, S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat. Rev. Cancer 2010, 10, 37–50. [Google Scholar]
- Burger, J.A.; Montserrat, E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood 2013, 121, 1501–1509. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, 201–241. [Google Scholar]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef]
- Rebacz, B.; Larsen, T.O.; Clausen, M.H.; Rønnest, M.H.; Löffler, H.; Ho, A.D.; Krämer, A. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 2007, 67, 6342–6350. [Google Scholar]
- Liao, W.-Y.; Shen, C.-N.; Lin, L.-H.; Yang, Y.-L.; Han, H.-Y.; Chen, J.-W.; Kuo, S.-C.; Wu, S.-H.; Liaw, C.-C. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terrus. J. Nat. Prod. 2012, 75, 630–635. [Google Scholar] [CrossRef]
- Schümann, J.; Hertweck, C. Molecular basis of cytochalasan biosynthesis in fungi: Gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J. Am. Chem. Soc. 2007, 129, 9564–9565. [Google Scholar] [CrossRef]
- Wagenaar, M.M.; Corwin, J.; Strobel, G.; Clardy, J. Three new cytochalasins produced by an endophytic fungus in the genus Rhinocladiella. J. Nat. Prod. 2000, 63, 1692–1695. [Google Scholar] [CrossRef]
- Liu, R.; Gu, Q.; Zhu, W.; Cui, C.; Fan, G.; Fang, Y.; Zhu, T.; Liu, H. 10-Phenyl-[12]-cytochalasins Z7, Z8, and Z9 from the marine-derived fungus Spicaria elegans. J. Nat. Prod. 2006, 69, 871–875. [Google Scholar] [CrossRef]
- Knudsen, P.B.; Hanna, B.; Ohl, S.; Sellner, L.; Zenz, T.; Döhner, H.; Stilgenbauer, S.; Larsen, T.O.; Lichter, P.; Seiffert, M. Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton. Leukemia 2014, 28, 1289–1298. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Pei, Y.; Hua, H.; Feng, B. Aspergillin PZ, a novel isoindole-alkaloid from Aspergillus awamori. J. Antibiot. (Tokyo) 2002, 55, 693–695. [Google Scholar] [CrossRef]
- Canham, S.M.; Overman, L.E.; Tanis, P.S. Identification of an Unexpected 2-Oxonia[3,3]sigmatropic Rearrangement/Aldol Pathway in the Formation of Oxacyclic Rings. Total Synthesis of (+)-Aspergillin PZ. Tetrahedron 2011, 67, 9837–9843. [Google Scholar] [CrossRef]
- Naruse, N.; Yamamoto, S.; Yamamoto, H. β-cyanoglutamic acid, a new antifungal amino acid from a streptomycete. J. Antibiot. (Tokyo) 1993, 46, 685–686. [Google Scholar] [CrossRef]
- Fang, F.; Ui, H.; Shiomi, K.; Masuma, R.; Yamaguchi, Y.; Zhang, C.G.; Zhang, X.W.; Tanaka, Y.; Omura, S. Two new components of the aspochalasins produced by Aspergillus sp. J. Antibiot. (Tokyo) 1997, 50, 919–925. [Google Scholar] [CrossRef]
- Choo, S.-J.; Yun, B.-S.; Ryoo, I.-J.; Kim, Y.-H.; Bae, K.-H.; Yoo, I.-D. Aspochalasin I, a Melanogenesis Inhibitor from Aspergillus sp. J. Microbiol. Biotechnol. 2009, 19, 368–371. [Google Scholar] [CrossRef]
- Zhou, G.-X.; Wijeratne, K.E.M.; Bigelow, D.; Pierson, L.S.; VanEtten, H.D.; Gunatilaka, L.A.A. Aspochalasins I, J, and K: Three new cytotoxic cytochalasans of Aspergillus flavipes from the rhizosphere of Ericameria laricifolia of the Sonoran Desert. J. Nat. Prod. 2004, 67, 328–332. [Google Scholar]
- Rochfort, S.; Ford, J.; Ovenden, S.; George, S.; Wildman, H.; Tait, R.M.; Meurer-Grimes, B.; Coxd, S.; Coatesd, J.; Rhodes, D. A novel aspochalasin with HIV-1 integrase inhibitory activity from Aspergillus flavipes. J. Antibiot. (Tokyo) 2005, 58, 279–283. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.; Huang, H.; Zheng, Z.; Xu, Q. Aspochalasin U, a moderate TNF-α inhibitor from Aspergillus sp. J. Antibiot. (Tokyo) 2012, 65, 49–52. [Google Scholar] [CrossRef]
- Kohno, J.; Nonaka, N.; Nishio, M.; Ohnuki, T.; Kawano, K.; Okuda, T.; Komatsubara, S. TMC-169, a new antibiotic of the aspochalasin group produced by Aspergillus flavipes. J. Antibiot. (Tokyo) 1999, 52, 575–577. [Google Scholar] [CrossRef]
- Gebhardt, K.; Schimana, J.; Holitzel, A.; Dettner, K.; Draeger, S.; Beil, W.; Rheinheimer, J.; Fiedler, H.-P. Aspochalamins A-D and aspochalasin Z produced by the endosymbiotic fungus Aspergillus niveus LU 9574. J. Antibiot. (Tokyo) 2004, 57, 707–714. [Google Scholar] [CrossRef]
- Barrow, C.; Sedlock, D.; Sun, H.; Cooper, R.; Gillum, A.M. WIN 66306, a new neurokinin antagonist produced by an Aspergillus species: Fermentation, isolation and physico-chemical properties. J. Antibiot. (Tokyo) 1994, 47, 1182–1187. [Google Scholar] [CrossRef]
- Fujishima, T.; Ichikawa, M.; Ishige, H.; Yoshino, H.; Ohishi, J.; Ikegami, S. Production of cytochalasin E by Aspergillus terreus. Hakkokogaku Kaishi–J. Soc. Ferment. Technol. 1979, 57, 15–19. [Google Scholar]
- Lin, Z.; Zhang, G.; Zhu, T.; Liu, R.; Wei, H.-J.; Gu, Q.-Q. Bioactive cytochalasins from Aspergillus flavipes, an endophytic fungus associated with the mangrove plant Acanthus ilicifolius. Helv. Chim. Acta 2009, 92, 1538–1544. [Google Scholar] [CrossRef]
- Ge, H.M.; Peng, H.; Guo, Z.K.; Cui, J.T.; Song, Y.C.; Tan, R.X. Bioactive alkaloids from the plant endophytic fungus Aspergillus terreus. Planta Med. 2010, 76, 822–824. [Google Scholar]
- Zhang, H.-W.; Zhang, J.; Hu, S.; Zhang, Z.-J.; Zhu, C.-J.; Ng, S.W.; Tan, R.-X. Ardeemins and cytochalasins from Aspergillus terreus residing in Artemisia annua. Planta Med. 2010, 76, 1616–21. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, H.; Wu, N.; Liu, M.; Wei, J.; Zhang, Y.; Lin, X. Characterization of the high cytochalasin E and rosellichalasin producing-Aspergillus sp. nov. F1 isolated from marine solar saltern in China. World J. Microbiol. Biotechnol. 2013, 29, 11–17. [Google Scholar]
- Keller-Schierlein, W.; Kupfer, E. Metabolites of microorganisms. 186. The aspochalasins A, B, C, and D. Helv. Chim. Acta 1979, 62, 1501–1524. [Google Scholar] [CrossRef]
- Tomikawa, T.; Kazuo, S.; Seto, H.; Okusa, N.; Kajiura, T.; Hayakawa, Y. Structure of aspochalasin H, a new member of the aspochalasin family. J. Antibiot. (Tokyo) 2002, 55, 666–668. [Google Scholar]
- Zheng, C.-J.; Shao, C.-L.; Wu, L.-Y.; Chen, M.; Wang, K.-L.; Zhao, D.-L.; Sun, X.-P.; Chen, G.-Y.; Wang, C.-Y. Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Mar. Drugs 2013, 11, 2054–2068. [Google Scholar] [CrossRef]
- Büchi, G.; Kitaura, Y.; Yuan, S. Structure of cytochalasin E, a toxic metabolite of Aspergillus clavatus. J. Am. Chem. Soc. 1973, 95, 5423–5425. [Google Scholar] [CrossRef]
- Steyn, P.S.; van Heerden, F.R.; Rabie, C. Cytochalasin-E and cytochalasin-K, toxic metabolites from Aspergillus clavatus. J. Am. Chem. Soc. Perkin 1 1982, 541–544. [Google Scholar] [CrossRef]
- Binder, M.; Tarnrn, C. Proxiphomin and Protophomin, 2 new cytochalasanes. Helv. Chim. Acta 1973, 7, 2387–2396. [Google Scholar] [CrossRef]
- Samson, R.; Houbraken, J.; Kuijpers, A. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 2004, 2, 45–61. [Google Scholar]
- Laatsch, H. Antibase 2012. Available online: http://www.wiley-vch.de/stmdata/antibase.php (accessed on 1 February 2014).
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS-KNAW Fungal Biodiversity Centre Utrecht: Utrecht, The Netherlands, 2010; pp. 372–374. [Google Scholar]
- Varga, J.; Frisvad, J.C.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Szigeti, G.; Samson, R.A. New and revisited species in Aspergillus section Nigri. Stud. Mycol. 2011, 69, 1–17. [Google Scholar] [CrossRef]
- Jurjević, Z.; Peterson, S.W.; Stea, G.; Solfrizzo, M.; Varga, J.; Hubka, V.; Perrone, G. Two novel species of Aspergillus section Nigri from indoor air. IMA Fungus 2012, 3, 159–173. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, L.M.; Bladt, T.T.; Dürr, C.; Seiffert, M.; Frisvad, J.C.; Gotfredsen, C.H.; Larsen, T.O. Isolation, Structural Analyses and Biological Activity Assays against Chronic Lymphocytic Leukemia of Two Novel Cytochalasins — Sclerotionigrin A and B. Molecules 2014, 19, 9786-9797. https://doi.org/10.3390/molecules19079786
Petersen LM, Bladt TT, Dürr C, Seiffert M, Frisvad JC, Gotfredsen CH, Larsen TO. Isolation, Structural Analyses and Biological Activity Assays against Chronic Lymphocytic Leukemia of Two Novel Cytochalasins — Sclerotionigrin A and B. Molecules. 2014; 19(7):9786-9797. https://doi.org/10.3390/molecules19079786
Chicago/Turabian StylePetersen, Lene M., Tanja T. Bladt, Claudia Dürr, Martina Seiffert, Jens C. Frisvad, Charlotte H. Gotfredsen, and Thomas O. Larsen. 2014. "Isolation, Structural Analyses and Biological Activity Assays against Chronic Lymphocytic Leukemia of Two Novel Cytochalasins — Sclerotionigrin A and B" Molecules 19, no. 7: 9786-9797. https://doi.org/10.3390/molecules19079786