Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts
Abstract
:1. Introduction

2. Results and Discussion
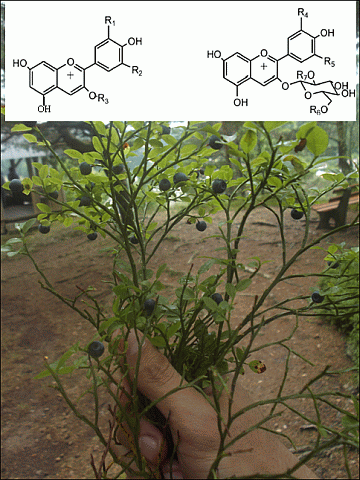
2.1. Accurate MS-PDA Identification of Anthocyanins in Six Small Berry Fruits from Southern Chile


| Peak Number | Retention Time (min) | Uv max | HR-M + ion (ppm) | Other ions (Aglycon moiety) | Formula | Identification | Fruit |
|---|---|---|---|---|---|---|---|
| 1 | 4.8 | 276–523 | 641.1687 (−4.8) | 317.0618 (Petunidin) | C28H33O17 | Petunidin-3-O-di-hexoside | blue |
| 2 | 5.9 | 280–517 | 611.1614 (0.3) | 449.1709 (Cyanidin-3-O-hexoside) | C27H31O16 | Cyanidin- 3-O-di-hexoside | blue, arr |
| 3 | 6.3 | 276–523 | 465.1040 (1.8) | 303.0500 (Delphinidin) | C21H21O12 | Delphinidin 3-O-galactoside * | blue, cal, che, arr, lu |
| 4 | 6.8 | 276–525 | 465.1038 (1.1) | 303.0495(Delphinidin) | C21H21O12 | Delphinidin 3-O-glucoside * | cal |
| 5 | 7.1 | 280–517 | 595.1478 (−31.0) | 449.1089 (Cyanidin-3-O-glucoside) | C27H31O15 | Cyanidin 3-O-rutinose | mu |
| 6 | 7.8 | 280–511 | 449.1052 (−7.1) | 287.0675 (Cyanidin) | C21H21O11 | Cyanidin-3-O-galactoside * | blue, che, lu |
| 7 | 9.1 | 280–517 | 449.1099 (3.3) | 287. 0507 (Cyanidin) | C21H21O11 | Cyanidin-3-O-glucoside * | blue, che, lu |
| 8 | 9.8 | 276–526 | 625.1789 (3.2) | 479.1198 (Petunidin-3-O-glucoside) | C28H33O16 | Petunidin-3-O-rutinoside | blue, cal, mu |
| 9 | 10.7 | 276–527 | 639.1911 (−2.2) | 493.1136 (Malvidin-3-O-glucoside) | C29H35O16 | Malvidin-3-O-rutinose | blue |
| 10 | 11.2 | 276–526 | 479.1233 (9.0) | 317.0672 (Petunidin) | C22H23O12 | Petunidin-3-O-glucoside * | blue, che, arr, lu |
| 11 | 11.9 | 276–523 | 479.1224 (7.1) | 317.0646 (Petunidin) | C22H23O12 | Petunidin-3-O-galactoside * | blue, cal, mu, che, lu |
| 12 | 12.5 | 276–525 | 609.1825 (0.8) | 301.0829 (Peonidin) | C28H33O15 | Peonidin 3-O-rutinose | blue |
| 13 | 12.7 | 279–520 | 463.1234 (−1.3) | 301.0689 (Peonidin) | C22H23O11 | Peonidin-3-O-galactoside * | blue |
| 14 | 13.4 | 276–527 | 493.1361 (3.0) | 331.0832 (Malvidin) | C23H25O12 | Malvidin-3-O-galactoside * | blue, arr |
| 15 | 14.0 | 276–523 | 435.0936 (2.1) | 303.0472 (Delphinidin) | C20H19O11 | Delphinidin-3-O-arabinoside | blue, cal |
| 16 | 14.7 | 276–527 | 463.1258 (3.9) | 301.1257 (Peonidin) | C22H23O11 | Peonidin-3-O-glucoside | cal, mu, arr |
| 17 | 15.3 | 276–527 | 493.1252 (−19.0) | 331.0789 (Malvidin) | C23H25O12 | Malvidin-3-O-glucoside * | blue, cal, arr, lu |
| 18 | 15.6 | 279–527 | 433.1131 (−0.92) | 301.0709 (Peonidin) | C21H21O10 | Peonidin-3-O-arabinoside | cal, mu |
| 19 | 16.2 | 276–526 | 449.1066 (−4.0) | 317.1969 (Petunidin) | C21H21O11 | Petunidin-3-O-arabinoside | blue |
| 20 | 16.7 | 280–517 | 419.0978 (−1.9) | 287. 0696 (Cyanidin) | C20H19O10 | Cyanidin-3-O-arabinoside * | blue |
| 21 | 17.3 | 276–311–527 | 639.1933 (34.2) | 493.1382 (Malvidin-3-O-glucoside) | C32H31O14 | Malvidin 3-O-(6ꞌꞌ coumaroyl) glucoside | cal |
| 22 | 17.8 | 276–527 | 463.1284 (9.5) | 330.1706 (Malvidin) | C22H23O11 | Malvidin-3-O-arabinose * | blue |
| 23 | 18.0 | 280–517 | 549.1639 (7.1) | 449.1082 (Cyanidin-3-O-glucose) | C25H25O14 | Cyanidin-3-O-(6ꞌꞌ succinoyl)-glucose | mu |
| 24 | 18.6 | 279–523 | 625.1820 (8.2) | 463.0905 (Peonidin-3-O- hexoside) | C28H33O16 | Peonidin 3-O-di hexoside | cal |
| 25 | 19.4 | 276–311–523 | 919.4460 (2.1) | 303.0504 (Delphinidin) | C42H47O23 | Delphinidin-3-O-rutinose (6ꞌꞌ-p-coumaroyl)-2ꞌꞌ-O-glucose | blue |
| 26 | 20.0 | 276–523 | 507.1135 (−0.4) | 303.0495 (Delphinidin) | C23H23O13 | Delphinidin 3-O-(6ꞌꞌ acetyl) glucoside | blue |
| 27 | 20.6 | 280–517 | 491.1206 (3.6) | 287.1232 (Cyanidin) | C23H23O12 | Cyanidin 3-O-(6ꞌꞌ acetyl) glucoside | blue |
| 28 | 21.4 | 276–526 | 521.1293 (−0.4) | 317.0676 (Petunidin) | C24H25O13 | Petunidin 3-O-(6ꞌꞌ acetyl) glucoside | blue, cal |
| 29 | 22.3 | 276–527 | 535.1451 (−0.2) | 331.0789 (Malvidin) | C25H27O13 | Malvidin 3-O-(6ꞌꞌ acetyl) galactoside | cal, arr |
| 30 | 23.2 | 276–321–523 | 627.1393 (−6.8) | 287.0743 (Cyanidin) | C30H27O15 | Delphinidin-3-O-(6ꞌꞌcaffeoyl)-glucose | blue |
| 31 | 24.0 | 276–527 | 535.1463 (1.5) | 331.0673 (Malvidin) | C25H27O13 | Malvidin 3-O-(6ꞌꞌ acetyl) glucoside | blue |
2.2. Identification of Phenolic Acids and Flavonols
2.3. Total Phenolics, Flavonoids and Anthocyanin Contents
| Species | DPPH α | FRAP β | SA ο | TPC δ | TFC ψ | TAC χ | Extraction Yields (%) µ |
|---|---|---|---|---|---|---|---|
| Vaccinium corymbosum | 3.32 ± 0.18 a | 96.15 ± 5.39 df | 72.61 ± 1.91 r | 45.86 ± 3.46 | 18.50 ± 3.75 p | 21.41 ± 1.65 | 6.72 |
| Berberis microphylla | 2.33 ± 0.21 ab | 124.46 ± 6.54 | 81.31 ± 2.95 s | 65.53 ± 1.35 | 45.72 ± 2.68 | 51.62 ± 1.78 | 4.99 |
| Luma chequén | 12.92 ± 0.30 | 76.22 ± 3.45 e | 43.79 ± 2.91 t | 5.11 ± 0.18 k | 2.57 ± 0.11 m | 1.54 ± 0.05 | 7.39 |
| Luma apiculata | 5.88 ± 0.21 | 93.4± 4.68 dg | 64.22 ± 3.46 | 27.61± 1.61 | 12.80± 2.43 np | 15.24 ± 1.49 l | 6.34 |
| Ugni molinae | 10.94 ± 0.32 c | 81.10 ± 4.58 ehj | 52.22 ± 1.81 t | 9.24 ± 0.28 k | 5.54 ± 0.91 mo | 6.85 ± 0.10 | 5.21 |
| Amomyrtus meli | 7.46 ± 0.10 b | 88.29 ± 6.34 fghi | 56.44 ± 2.32 | 17.52 ± 0.66 | 11.76 ± 2.04 no | 13.33 ±2.69 l | 4.89 |
| Gallic acid ϕ | 1.36 ± 0.22 (7.99 ± 1.29 μM) | 148.1 ± 8.35 | 94.39 ± 1.98 | - | - | - | - |
| Cyanidin 3-O-glucoside ϕ | 8.47 ± 1.23 c (17.47 ± 2.53 µM) | 95.48 ± 6.72 ij | 76.85 ± 1.71 rs | - | - | - | - |
2.4. Quantification of Individual Anthocyanins
2.5. Antioxidant Features

| Anthocyanin (mg/100 g) a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berry Species | 2 | 3 | 6 | 7 | 8 | 10 | 11 | 13 | 15 | 16 | 17 | 18 | 20 | Total |
| Vaccinium corymbosum | 19.23 ± 3.18 | 8.51 ± 3.29 a | 21.17 ± 0.32 | 0.96 ± 0.12 | 14.29 ± 2.15 | 9.27 ± 2.22 d | 1.78 ± 0.01 | 14.28 ± 0.98 | 34.43 ± 3.28 | nd | 16.42 ± 1.45 | nd | 37.43 ± 4. 76 | 177.77 |
| Berberis microphylla | nd | 60.42 ± 1.28 | nd | 21.89 ± 2.74 b | nd | 51.39 ± 1.65 e | 6.45 ± 0.89 | nd | 9.28 ± 0.01 | 3.96 ± 0.02 | 42.94 ± 1.25 f | 3.84 ± 0.02 | nd | 200.17 |
| Luma chequén | nd | 3.72 ± 0.02 | 43.46 ± 1.39 | 5.29 ± 0.23 | nd | 12.83 ± 1.65 | 9.55 ± 1.02 | nd | nd | nd | nd | nd | nd | 74.85 |
| Luma apiculata | 2.6 ± 0.01 | 34.43 ± 2.12 | nd | 9.45 ± 0.15 | nd | 48.21 ± 2.2 e | nd | nd | nd | nd | 44.75 ± 3.31 f | nd | nd | 139.44 |
| Ugni molinae | nd | nd | nd | nd | 51.37 ± 0.28 | nd | 4.87 ± 0.02 | nd | nd | 61.48 ± 2.42 | nd | 4.43 ± 0.04 | nd | 122.15 |
| Amomyrtus meli | nd | 8.87 ± 1.76a | 48.39 ± 2.23 | 20.43 ± 2.39 b | 13.54 ± 2.46 d | 27.12 ± 1.25 | nd | nd | nd | 8.45 ±1.13 | nd | nd | 126.80 | |
| Inter day | Intra day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Nominal concentration (μg/mL) | Observed concentration (μg/mL) | Accuracy (%) | RSD% | Observed concentration (μg/mL) | Accuracy (%) | RSD% | LOD-LOQ (μg/mL) | Sample-Recovery (mean ± RSD% ) |
| 3 | 10 | 10.94 ± 0.05 | 109.45 | 0.071 | 11.83 ± 0.05 | 118.33 | 0.04 | 0.08–0.24 | Calafate 97.93 ± 0.33 |
| 3 | 20 | 20.91 ± 0.62 | 104.55 | 0.80 | 21.73 ± 0.37 | 108.66 | 0.34 | Arrayán 98.73 ± 1.50 | |
| 3 | 40 | 40.49 ± 0.70 | 101.23 | 0.69 | 41.2 ± 0.72 | 103.00 | 0.70 | Blueberry 98.57 ± 0.33 | |
| 7 | 10 | 12.0 ± 0.08 | 120.00 | 0.08 | 12.13 ± 0.15 | 121.33 | 0.12 | 0.12–0.35 | Calafate 99.72 ± 1.34 |
| 7 | 20 | 19.81± 0.27 | 99.08 | 0.28 | 20.36 ± 0.76 | 101.80 | 0.75 | Arrayán 98.97 ± 1.98 | |
| 7 | 40 | 40.77 ± 0.37 | 101.93 | 0.36 | 40.46 ± 0.67 | 101.15 | 0.66 | Chequén 99.84 ± 0.16 | |
| 10 | 10 | 10.56 ± 0.81 | 105.66 | 0.77 | 9.99 ± 0.12 | 99.96 | 0.12 | 0.09–0.30 | Calafate 99.31 ± 0.35 |
| 10 | 20 | 20.20 ± 0.59 | 101.03 | 0.58 | 20.61 ± 0.33 | 103.05 | 0.32 | Arrayán 98.59 ± 0.38 | |
| 10 | 40 | 41.04 ± 0.41 | 102.64 | 0.40 | 40.8 ± 0.36 | 40.8 | 0.79 | Chequén 98.19 ± 0.76 |
3. Experimental
3.1. Chemicals and Plant Material
3.2. Sample Preparation
3.3. Liquid Chromatography Analysis
3.4. Validation of the HPLC Method
3.5. Mass Spectrometric Conditions
3.6. Antioxidant Assays
3.6.1. Free Radical Scavenging Capacity
3.6.2. Ferric Reducing Antioxidant Power
3.6.3. Superoxide Anion Scavenging Activity
3.6.4. Polyphenol, Flavonoids and Anthocyanin Contents

3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetable subgroups. J. Food Compos. Anal. 2010, 23, 411–418. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Fedirko, V.; Trichopoulou, A.; Gonzalez, C.A.; Bamia, C.; Trepo, E.; Nothlings, U.; Duarte-Salles, T.; Serafini, M.; Bredsdorff, L.; et al. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2013, 133, 2429–2443. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. F. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some vegetables and their role in human nutrition. Hortic. Sci. 2000, 27, 65–78. [Google Scholar]
- Delporte, C.; Backhouse, N.; Inostroza, V.; Aguirre, M.C.; Peredo, N.; Silva, X.; Negrete, R.; Miranda, H.F. Analgesic activity of Ugni molinae (murtilla) in mice models of acute pain. J. Ethnopharmacol. 2007, 112, 162–165. [Google Scholar] [CrossRef]
- Scheuermann, E.; Seguel, I.; Montenegro, A.; Bustos, R.; Hormazabal, E.; Quiroz, A. Evolution of aroma compounds of murtilla fruits (Ugni molinale Turcz) during storage. J. Sci. Food Agric. 2008, 88, 485–492. [Google Scholar] [CrossRef]
- Murillo, A. Plantes médicinales du Chili; Exposition Universelle de Paris Section Chilienne; Roger y Chernoviz: Paris, France, 1889; Volum 80, p. 234. [Google Scholar]
- Hoffmann, A.E. Flora Silvestre de Chile, 3rd ed.; Gay, C., Ed.; Ediciones Fundacion Claudio Gay: Santiago, Chile, 1995; Volume 1, p. 258. [Google Scholar]
- De Mösbach, E.W. Botánica indígena de Chile. In Museo Chileno de Arte Precolombino, Fundación Andes y Editorial Andrés Bello; Aldunate, C., Villagrán, C., Eds.; Editorial Andrés Bello: Santiago, Chile, 1991; pp. 95–96. [Google Scholar]
- Mariangel, E.; Reyes-Diaz, M.; Lobos, W.; Bensch, E.; Schalchli, H.; Ibarra, P. The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc. Investig. Agrar. 2013, 40, 161–170. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of Calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A 2013, 1281, 38–45. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Yang, H.; Jiang, B.; Basile, M.J.; Kennelly, E.J. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008, 109, 883–890. [Google Scholar] [CrossRef]
- Theoduloz, C.; Franco, L.; Ferro, E.; Schmeda Hirschmann, G. Xanthine oxidase inhibitory activity of Paraguayan Myrtaceae. J. Ethnopharmacol. 1988, 24, 179–183. [Google Scholar] [CrossRef]
- Theoduloz, C.; Pacheco, P.; Schmeda Hirschmann, G. Xanthine oxidase inhibitory activity of Chilean Myrtaceae. J. Ethnopharmacol. 1991, 33, 253–255. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Borquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequen. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef]
- Bórquez, J.; Kennelly, E.J.; Simirgiotis, M.J. Activity guided isolation of isoflavones and hyphenated HPLC-PDA-ESI-ToF-MS metabolome profiling of Azorella madreporica Clos. from northern Chile. Food Res. Int. 2013, 52, 288–297. [Google Scholar] [CrossRef]
- Wu, S.-B.; Wu, J.; Yin, Z.; Zhang, J.; Long, C.; Kennelly, E.J.; Zheng, S. Bioactive and Marker Compounds from Two Edible Dark-Colored Myrciaria Fruits and the Synthesis of Jaboticabin. J. Agric. Food Chem. 2013, 61, 4035–4043. [Google Scholar] [CrossRef]
- Wu, S.-B.; Dastmalchi, K.; Long, C.; Kennelly, E.J. Metabolite Profiling of Jaboticaba (Myrciaria cauliflora) and Other Dark-Colored Fruit Juices. J. Agric. Food Chem. 2012, 60, 7513–7525. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography–electrospray ionization-time of flight-mass spectrometry (RP-HPLC–ESI-TOF-MS). J.Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhu, Q.; Wang, D.; Wang, W.; Sun, X.; Liu, X.; Du, F. Screening natural antioxidants in peanut shell using DPPH–HPLC–DAD–TOF/MS methods. Food Chem. 2012, 135, 2366–2371. [Google Scholar] [CrossRef]
- Lätti, A.K.; Riihinen, K.R.; Jaakola, L. Phenolic compounds in berries and flowers of a natural hybrid between bilberry and lingonberry (Vaccinium intermedium Ruthe). Phytochemistry 2011, 72, 810–815. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Santos, L.S.; Torres, S. Anthocyanin composition in aged Chilean Cabernet Sauvignon red wines. Food Chem. 2011, 129, 514–519. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography−electrospray Ionization−tandem mass spectrometry in common foods in the United States: vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Yousef, G.G.; Brown, A.F.; Funakoshi, Y.; Mbeunkui, F.; Grace, M.H.; Ballington, J.R.; Loraine, A.; Lila, M.A. Efficient quantification of the health-relevant anthocyanin and phenolic acid profiles in commercial cultivars and breeding selections of Blueberries (Vaccinium spp.). J. Agric. Food Chem. 2013, 61, 4806–4815. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef]
- Schierle, J.; Pietsch, B.; Ceresa, A.; Fizet, C. Method for the determination of β-carotene in supplements and raw materials by reversed-phase liquid chromatography: Single laboratory validation. J. AOAC Int. 2004, 87, 1070–1082. [Google Scholar]
- Bunea, A.; Rugina, D.; Sconta, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tabaran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Lo Scalzo, R.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal.Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography-UV detection-mass spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Sample Availability: Samples of the compounds and extract of the berries are available from authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules 2014, 19, 10936-10955. https://doi.org/10.3390/molecules190810936
Brito A, Areche C, Sepúlveda B, Kennelly EJ, Simirgiotis MJ. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules. 2014; 19(8):10936-10955. https://doi.org/10.3390/molecules190810936
Chicago/Turabian StyleBrito, Anghel, Carlos Areche, Beatriz Sepúlveda, Edward J. Kennelly, and Mario J. Simirgiotis. 2014. "Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts" Molecules 19, no. 8: 10936-10955. https://doi.org/10.3390/molecules190810936