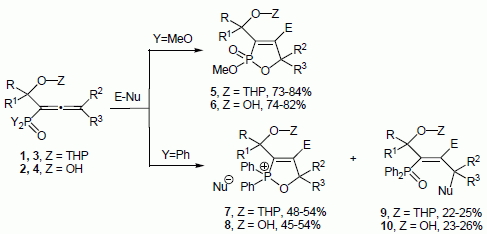
3. Experimental Section
3.2. Starting Materials
Diphenyl disulfide and sulfuryl chloride in dichloromethane and distilled
in vacuo (bp 80–81 °C/20 mm Hg) [
58] were used to prepare benzenesulfanyl chloride. Diphenyl disulfide, sulfuryl chloride, and benzeneselenenyl chloride were commercially available and used without purification. The starting phosphorylated α-hydroxyallenes
1–
4 were prepared according to the established procedure [
55].
3.3. General Procedure for the Reactions of the Dimethyl 1-(Tetrahydro-2H-pyran-2-yloxy)methyl-1,2-dienephosphonates 1 with Electrophilic Reagents
To a solution of the dimethyl 1-(tetrahydro-2
H-pyran-2-yloxy)methyl-1,2-dienephosphonates
1 (3.0 mmol) in dry dichloromethane (10 mL) at −20 °C was added dropwise with stirring a solution of electrophilic reagent (sulfuryl chloride, bromine or benzeneselenenyl chloride) (3.6 mmol) in the same solvent (10 mL). The reaction mixture was stirred at the same temperature for several h (see
Table 1) and an hour at room temperature. After evaporation of the solvent, the residue was chromatographed on a silica gel column (ethyl acetate and hexane 4:1) as eluent to give the pure products
5 as oils, which had the following properties:
2-[(4-Bromo-5-ethyl-2-methoxy-5-methyl-2-oxo-2,5-dihydro-1,2-oxaphosphol-3-yl)methoxy]-tetra-hydro-2H-pyran (5a). Yellow oil, yield: 81%. Rf 0.49; IR (neat, cm−1): 1015 (C-O-P), 1120 (C-O-C), 1268 (P=O), 1583 (C=C). 1H-NMR (250.1 MHz): δ 0.89 (t, J = 7.2 Hz, 3H, Me-CH2), 1.53 (s, 3H, Me-C), 1.54–1.89, 3.55–3.68, 4.53–4.63 (overlapping multiplets, 9H, OTHP), 1.77–1.88 (m, 2H, Me-CH2), 3.83 (d, J = 9.3 Hz, 3H, MeO), 3.91–4.07 (m, 2H, CH2O). 13C-NMR (62.9 MHz) δ = 9.3 (J = 4.7 Hz), 19.6, 24.6 (J = 7.7 Hz), 31.2, 31.5 (J = 7.9 Hz), 32.5 (J = 7.8 Hz), 53.4 (J = 13.9 Hz), 63.7, 65.8 (J = 5.7 Hz), 89.6 (J = 9.8 Hz), 97.1 (J = 5.0 Hz), 130.5 (J = 156.4 Hz), 140.7 (J = 51.4 Hz). 31P-NMR (101.2 MHz): δ 31.8. Anal. Calcd for C13H22BrO5P (369.19): C 42.29, H 6.01. Found: C 42.35, H 5.93.
2-[(4-Bromo-5-butyl-2-methoxy-5-methyl-2-oxo-2,5-dihydro-1,2-oxaphosphol-3-yl)methoxy]-tetra-hydro-2H-pyran (5b). Dark orange oil, yield: 80%. Rf 0.53; IR (neat, cm−1): 1012 (C-O-P), 1123 (C-O-C), 1263 (P=O), 1587 (C=C). 1H-NMR (600.1 MHz): δ 0.91 (t, J = 7.3 Hz, 3H, Me-CH2), 1.28–1.36, 1.49–1.60, 1.77–1.85 (overlapping multiplets, 6H, (CH2)3-Me), 1.51–1.58, 3.56–3.60, 4.54–4.63 (overlapping multiplets, 9H, OTHP), 1.56 (s, 3H, Me-C), 3.85 (d, J = 9.4 Hz, 3H, MeO), 3.89–3.99 (m, 2H, CH2O). 13C-NMR (150.9 MHz) δ = 14.1, 19.9, 22.6, 23.4 (J = 4.6 Hz), 25.1 (J = 7.9 Hz), 26.7, 32.5, 40.5 (J = 7.9 Hz), 53.2 (J = 14.2 Hz), 62.8, 64.9 (J = 5.6 Hz), 88.7 (J = 10.0 Hz), 96.5 (J = 5.1 Hz), 129.9 (J = 155.6 Hz), 141.5 (J = 52.1 Hz). 31P-NMR (242.9 MHz): δ 31.9. Anal. Calcd for C15H26 BrO5P (397.24): C 45.35, H 6.60. Found: C 45.29, H 6.56.
4-Chloro-2-methoxy-3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-1-oxa-2-phosphaspiro[4.5]dec-3-ene 2-oxide (5c). Yellow oil, yield: 83%. Rf 0.47; IR (neat, cm−1): 1019 (C-O-P), 1117 (C-O-C), 1261 (P=O), 1584 (C=C). 1H-NMR (250.1 MHz): δ 1.32–1.92, 2.14–2.23, 3.61–3.77, 4.53–4.59 (overlapping multiplets, 19H, (CH2)5, OTHP), 3.69 (d, J = 9.4 Hz, 3H, MeO), 3.95–4.07 (m, 2H, CH2O). 13C-NMR (62.9 MHz) δ = 19.5, 22.6 (J = 5.0 Hz), 24.1, 25.7, 31.7, 35.4 (J = 7.8 Hz), 36.5 (J = 7.7 Hz), 52.4 (J = 14.5 Hz), 62.4, 64.6 (J = 5.7 Hz), 87.2 (J = 9.5 Hz), 96.5 (J = 4.9 Hz), 129.5 (J = 156.4 Hz), 140.7 (J = 52.5 Hz). 31P-NMR (101.2 MHz): δ 32.4. Anal. Calcd for C15H24ClO5P (350.77): C 51.36, H 6.90. Found: C 51.43, H 6.96.
4-Bromo-2-methoxy-3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-1-oxa-2-phosphaspiro[4.5]dec-3-ene 2-oxide (5d). Dark orange oil, yield: 84%. Rf 0.51; IR (neat, cm−1): 1013 (C-O-P), 1117 (C-O-C), 1269 (P=O), 1581 (C=C). 1H-NMR (600.1 MHz): δ 1.29–1.68, 1.95–2.05, 2.28–2.36, 3.60–3.76, 4.52–4.57 (overlapping multiplets, 19H, (CH2)5, OTHP), 3.78 (d, J = 9.3 Hz, 3H, MeO), 3.93–4.06 (m, 2H, CH2O). 13C-NMR (150.9 MHz) δ = 19.3, 22.0 (J = 4.8 Hz), 23.9, 25.4, 31.5, 34.6 (J = 7.9 Hz), 37.1 (J = 7.9 Hz), 52.5 (J = 14.4 Hz), 62.2, 64.8 (J = 5.9 Hz), 87.1 (J = 9.7 Hz), 96.3 (J = 5.0 Hz), 129.2 (J = 156.0 Hz), 139.6 (J = 51.6 Hz). 31P-NMR (242.9 MHz): δ 33.0. Anal. Calcd for C15H24BrO5P (395.23): C 45.58, H 6.12. Found: C 45.63, H 6.19.
2-Methoxy-4-phenylselenenyl-3-[1-(tetrahydro-2H-pyran-2-yloxy)ethyl]-1-oxa-2-phosphaspiro[4.5]dec-3-ene 2-oxide (5e). Orange oil, yield: 74%. Rf 0.48; IR (neat, cm−1): 1011 (C-O-P), 1122 (C-O-C), 1259 (P=O), 1589 (C=C). 1H-NMR (600.1 MHz): δ 1.13–1.74, 2.01–2.09, 3.59–3.69, 4.63–4.68 (overlapping multiplets, 19H, (CH2)5, OTHP), 1.38 (dd, J = 10.6 Hz, J = 6.5 Hz, 3H, Me-CH), 3.72 (d, J = 9.2 Hz, 3H, MeO), 4.21–4.29 (m, 1H, Me-CH), 7.39–7.44 (m, 5H, Ph). 13C-NMR (150.9 MHz) δ = 19.4, 21.0 (J = 5.0 Hz), 21.3 (J = 7.8 Hz), 23.7, 25.6, 31.4, 34.1 (J = 7.8 Hz), 36.3 (J = 7.9 Hz), 51.9 (J = 14.7 Hz), 62.5, 76.2 (J = 6.1 Hz), 89.4 (J = 9.8 Hz), 95.2 (J = 4.9 Hz), 129.4-139.0, 131.4 (J = 105.4 Hz), 174.2 (J = 81.4 Hz). 31P-NMR (242.9 MHz): δ 34.5. Anal. Calcd for C22H31O5PSe (485.41): C 54.44, H 6.44. Found: C 54.40, H 6.52.
2[1-(5-Butyl-2-methoxy-5-methyl-2-oxo-4-phenylselenenyl-2,5-dihydro-1,2-oxaphosphol-3-yl)methyl-ethoxy]-tetrahydro-2H-pyran (5f). Yellow oil, yield: 73%. Rf 0.47; IR (neat, cm−1): 1014 (C-O-P), 1121 (C-O-C), 1266 (P=O), 1586 (C=C). 1H-NMR (600.1 MHz): δ 0.81 (t, J = 7.4 Hz, 3H, Me-CH2), 1.26–1.33, 1.39–1.46, 1.81–1.93 (overlapping multiplets, 6H, (CH2)3-Me), 1.52–1.70, 3.72–3.86, 4.71–4.76 (overlapping multiplets, 9H, OTHP), 1.56 (s, 3H, Me-C), 3.84 (d, J = 9.6 Hz, 3H, MeO), 1.48, 1.53 (ss, 6H, Me2C), 7.49–7.58 (m, 5H, Ph). 13C-NMR (150.9 MHz) δ = 14.2, 20.4, 23.1, 23.6 (J = 4.7 Hz), 24.7 (J = 8.0 Hz), 25.2, 29.9 (J = 7.9 Hz), 32.4, 39.7 (J = 8.1 Hz), 52.4 (J = 15.0 Hz), 63.7, 84.2 (J = 6.0 Hz), 91.7 (J = 10.0 Hz), 94.4 (J = 4.8 Hz), 128.7–138.7, 132.7 (J = 106.9 Hz), 175.4 (J = 82.8 Hz). 31P-NMR (242.9 MHz): δ 33.5. Anal. Calcd for C23H35O5PSe (501.45): C 55.09, H 7.04. Found: C 55.02, H 6.99.
3.4. General Procedure for the Reactions of the 1-Hydroxyalkyl-1,2-dienephosphonates 2 with Electrophilic Reagents
We got a solution of the 1-hydroxyalkyl-1,2-dienephosphonates
2 (3.0 mmol) where in dry dichloromethane (10 mL) at −20 °C was added dropwise with stirring a solution of electrophilic reagent (sulfuryl chloride, bromine, benzenesulfenyl chloride or benzeneselenenyl chloride) (3.6 mmol) in the same solvent (10 mL). The mixture was stirred at the same temperature for several h (see
Table 1) and an hour at room temperature. After evaporation of the solvent, the residue was chromatographed on a silica gel column (ethyl acetate and hexane 2:1) as eluent to give the pure products
6 as oils, which had the following properties:
(4-Bromo-5-ethyl-2-methoxy-5-methyl-2-oxo-2,5-dihydro-1,2-oxaphosphol-3-yl)-methanol (6a). Yellow oil, yield: 80%. Rf 0.56; IR (neat, cm−1): 1018 (C-O-P), 1263 (P=O), 1587 (C=C), 3413 (OH). 1H-NMR (600.1 MHz): δ 0.89 (t, J = 7.1 Hz, 3H, Me-CH2), 1.59 (s, 3H, Me-C), 1.78–1.99 (m, 2H, Me-CH2), 3.11 (s, 1H, OH), 3.79 (d, J = 9.6 Hz, 3H, MeO), 4.51–4.56 (m, 2H, CH2O). 13C-NMR (150.9 MHz) δ = 9.4 (J = 4.8 Hz), 24.3 (J = 7.8 Hz), 31.1 (J = 7.8 Hz), 52.7 (J = 14.3 Hz), 61.4 (J = 5.9 Hz), 91.4 (J = 9.9 Hz), 129.7 (J = 155.0 Hz), 140.4 (J = 50.7 Hz). 31P-NMR (242.9 MHz): δ 35.7. Anal. Calcd for C8H14BrO4P (285.07): C 33.71, H 4.95. Found: C 33.65, H 5.02.
(5-Butyl-2-methoxy-5-methyl-2-oxo-4-phenylsulfenyl-2,5-dihydro-1,2-oxaphosphol-3-yl)-methanol (6b). Orange oil, yield: 75%. Rf 0.49; IR (neat, cm−1): 1010 (C-O-P), 1260 (P=O), 1584 (C=C), 3409 (OH). 1H-NMR (600.1 MHz): δ 0.91 (t, J = 7.2 Hz, 3H, Me-CH2), 1.26–1.35, 1.60–1.64, 1.86–2.05 (overlapping multiplets, 6H, (CH2)3-Me), 1.47 (s, 3H, Me-C), 3.69 (s, 1H, OH), 3.75 (d, J = 9.5 Hz, 3H, MeO), 4.68–4.71 (m, 2H, CH2O), 7.16–7.44 (m, 5H, Ph). 13C-NMR (150.9 MHz) δ = 14.1, 23.2, 24.4 (J = 4.7 Hz), 27.8 (J = 7.9 Hz), 40.4 (J = 7.8 Hz), 51.9 (J = 14.6 Hz), 61.6 (J = 6.0 Hz), 88.9 (J = 9.8 Hz), 126.7–135.8, 128.6 (J = 102.0 Hz), 158.1 (J = 51.2 Hz). 31P-NMR (242.9 MHz): δ 33.1. Anal. Calcd for C16H23O4PS (342.39): C 56.13, H 6.77. Found: C 56.19, H 6.84.
(2-Methoxy-2-oxo-4-phenylselenenyl-1-oxa-phospha-spiro[4.5]dec-3-en-3-yl)-methanol (6c). Orange oil, yield: 74%. Rf 0.48; IR (neat, cm−1): 1018 (C-O-P), 1268 (P=O), 1580 (C=C), 3418 (OH). 1H-NMR (250.1 MHz): δ 1.16–1.39, 1.60–1.79, 1.84–2.07 (overlapping multiplets, 10H, (CH2)5), 3.75 (s, 1H, OH), 3.78 (d, J = 9.8 Hz, 3H, MeO), 4.66–4.69 (m, 2H, CH2O), 7.28–7.37 (m, 5H, Ph). 13C-NMR (62.9 MHz) δ = 21.4 (J = 4.9 Hz), 23.9, 33.9 (J = 7.8 Hz), 36.2 (J = 7.9 Hz), 52.4 (J = 14.7 Hz), 60.9 (J = 6.0 Hz), 89.4 (J = 9.8 Hz), 127.4 (J = 106.0 Hz), 127.6–137.9, 174.2 (J = 82.4 Hz). 31P-NMR (101.2 MHz): δ 36.3. Anal. Calcd for C16H21O4PSe (387.27): C 49.62, H 5.47. Found: C 49.56, H 5.51.
1-(4-Chloro-2-methoxy-2-oxo-1-oxa-phospha-spiro[4.5]dec-3-en-3-yl)-ethanol (6d). Yellow oil, yield: 82%. Rf 0.54; IR (neat, cm−1): 1011 (C-O-P), 1259 (P=O), 1583 (C=C), 3424 (OH). 1H-NMR (250.1 MHz): δ 1.33–1.48, 1.64–1.85, 1.94–2.14 (overlapping multiplets, 10H, (CH2)5), 1.48 (dd, J = 10.5 Hz, J = 6.4 Hz, 3H, Me-CH), 3.67 (d, J = 9.4 Hz, 3H, MeO), 3.90 (s, 1H, OH), 4.69–4.78 (m, 1H, Me-CH). 13C-NMR (62.9 MHz) δ = 22.4 (J = 5.0 Hz), 24.1, 24.5 (J = 7.9 Hz), 34.4 (J = 7.9 Hz), 36.8 (J = 7.9 Hz), 51.9 (J = 15.1 Hz), 72.6 (J = 5.8 Hz), 90.5 (J = 10.1 Hz), 129.3 (J = 101.6 Hz), 160.6 (J = 40.7 Hz). 31P-NMR (101.2 MHz): δ 35.7. Anal. Calcd for C11H18ClO4P (280.68): C 47.07, H 6.46. Found: C 46.99, H 6.40.
2-(4-Bromo-5-butyl-2-methoxy-5-methyl-2-oxo-2,5-dihydro-1,2-oxaphosphol-3-yl)-propan-2-ol (6e). Dark orange oil, yield: 81%. Rf 0.51; IR (neat, cm−1): 1009 (C-O-P), 1268 (P=O), 1589 (C=C), 3410 (OH). 1H-NMR (600.1 MHz): δ 0.91 (t, J = 7.3 Hz, 3H, Me-CH2), 1.27–1.34, 1.50–1.57, 1.76–1.92 (overlapping multiplets, 6H, (CH2)3-Me), 1.48 (s, 3H, Me-C), 1.56, 1.58 (ss, 6H, Me2C), 3.48 (s, 1H, OH), 3.65 (d, J = 9.5 Hz, 3H, MeO). 13C-NMR (150.9 MHz) δ = 14.2, 23.1, 23.5 (J = 4.6 Hz), 25.4 (J = 7.9 Hz), 31.4 (J = 8.1 Hz), 39.4 (J = 7.9 Hz), 52.3 (J = 15.0 Hz), 71.4 (J = 6.0 Hz), 92.4 (J = 9.8 Hz), 133.1 (J = 154.3 Hz), 142.8 (J = 51.4 Hz). 31P-NMR (242.9 MHz): δ 34.1. Anal. Calcd for C12H22BrO4P (341.18): C 42.24, H 6.50. Found: C 42.31, H 6.56.
3.5. General Procedure for the Reactions of the 2-[2-(Diphenylphosphinoyl)-2,3-dienyloxy]methyl-tetrahydro-2H-pyrans 3 with Electrophilic Reagents
To a solution of the 2-[2-(diphenylphosphinoyl)-2,3-dienyloxy]methyl-tetrahydro-2
H-pyrans
3 (3.0 mmol) in dry dichloromethane (10 mL) at −20 °C was added dropwise with stirring a solution of electrophilic reagent (sulfuryl chloride, bromine or benzeneselenenyl chloride) (3.6 mmol) in the same solvent (10 mL). The reaction mixture was stirred at the same temperature for several h (see
Table 2) and an hour at room temperature. The solvent was removed using a rotatory evaporator and the residue was purified by column chromatography (silica gel, ethyl acetate and hexane 4:1). The pure products
7 and
9 had the following properties:
4-Bromo-5-ethyl-5-methyl-2,2-diphenyl-3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-2,5-dihydro-1,2-oxaphosphol-2-ium bromide (7a). Orange oil, yield: 50%. Rf 0.38; IR (neat, cm−1): 1119 (C-O-C), 1439, 1484 (Ph), 1583 (C=C). 1H-NMR (600.1 MHz): δ 1.07 (t, J = 7.1 Hz, 3H, Me-CH2), 1.44–1.69, 3.61–3.75, 4.80–4.85 (overlapping multiplets, 9H, OTHP), 1.78 (s, 3H, Me-C), 2.28–2.37 (m, 2H, Me-CH2), 4.31–4.51 (m, 2H, CH2O), 7.75–8.46 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 7.9 (J = 4.5 Hz), 19.4, 25.4, 27.1 (J = 7.9 Hz), 31.2, 31.6 (J = 8.0 Hz), 62.6 (J = 7.8 Hz), 63.0, 92.4 (J = 10.1 Hz), 98.4 (J = 4.6 Hz), 111.4–135.2, 134.7 (J = 51.2 Hz), 158.7 (J = 50.9 Hz). 31P-NMR (242.9 MHz): δ 86.5. Anal. Calcd for C24H29Br2O3P (556.27): C 51.82, H 5.25. Found: C 51.74, H 5.20.
(1E)-2,3-Dibromo-3-methyl-1-[(tetrahydro-2H-pyran-2-yloxy)methyl]pent-1-en-1-yl diphenyl phosphine oxide (9a). Colourless oil, yield: 23%. Rf 0.62; IR (neat, cm−1): 1121 (C-O-C), 1153 (P=O), 1435, 1488 (Ph), 1618 (C=C). 1H-NMR (600.1 MHz): δ 1.12 (t, J = 7.3 Hz, 3H, Me-CH2), 1.46–1.71, 3.62–3.77, 4.51–4.57 (overlapping multiplets, 9H, OTHP), 1.98–2.20 (m, 2H, Me-CH2), 2.16 (s, 3H, Me-C), 3.91–4.07 (m, 2H, CH2O), 7.53–8.12 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 9.4, 19.6, 25.3, 30.8, 35.4 (J = 4.6 Hz), 36.4 (J = 5.0 Hz), 62.4, 62.6 (J = 7.9 Hz), 68.4 (J = 5.8 Hz), 96.3 (J = 5.0 Hz), 129.3–133.3, 132.4 (J = 154.7 Hz), 141.7 (J = 50.4 Hz). 31P-NMR (242.9 MHz): δ 39.7. Anal. Calcd for C24H29Br2O3P (556.27): C 51.82, H 5.25. Found: C 51.87, H 5.17.
4-Bromo-5-butyl-5-methyl-2,2-diphenyl-3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-2,5-dihydro-1,2-oxaphosphol-2-ium bromide (7b). Orange oil, yield: 48%. Rf 0.37; IR (neat, cm−1): 1120 (C-O-C), 1434, 1489 (Ph), 1588 (C=C). 1H-NMR (600.1 MHz): δ 0.91 (t, J = 6.3 Hz, 3H, Me-CH2), 1.08–1.15, 1.28–1.38, 2.26–2.44 (overlapping multiplets, 6H, (CH2)3-Me), 1.46–1.70, 3.58–3.72, 4.77–4.81 (overlapping multiplets, 9H, OTHP), 1.77 (s, 3H, Me-C), 4.35–4.49 (m, 2H, CH2O), 7.73–8.50 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 14.4, 19.5, 23.0, 23.4 (J = 4.5 Hz), 25.2, 27.3 (J = 8.0 Hz), 31.4, 39.7 (J = 7.7 Hz), 62.3, 62.8 (J = 7.5 Hz), 92.2 (J = 9.8 Hz), 98.3 (J = 4.7 Hz), 110.2-134.8, 133.6 (J = 49.5 Hz), 159.8 (J = 51.2 Hz). 31P-NMR (242.9 MHz): δ 86.6. Anal. Calcd for C26H33Br2O3P (584.32): C 53.44, H 5.69. Found: C 53.37, H 5.73.
(1E)-2,3-Dibromo-3-methyl-1-[(tetrahydro-2H-pyran-2-yloxy)methyl]hept-1-en-1-yl diphenyl phosphine oxide (9b). Yellow oil, yield: 22%. Rf 0.64; IR (neat, cm−1): 1119 (C-O-C), 1163 (P=O), 1439, 1484 (Ph), 1612 (C=C). 1H-NMR (600.1 MHz): δ 0.85 (t, J = 6.3 Hz, 3H, Me-CH2), 1.30–1.43, 1.47–1.72 (overlapping multiplets, 6H, (CH2)3-Me), 2.01–2.21, 3.36–3.74, 4.52–4.58 (overlapping multiplets, 9H, OTHP), 2.14 (s, 3H, Me-C), 3.92–4.06 (m, 2H, CH2O), 7.51–8.13 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 14.4, 19.4, 22.3, 25.4, 27.3, 31.2, 35.2 (J = 4.7 Hz), 43.2 (J = 5.1 Hz), 58.9 (J = 7.8 Hz), 62.4, 67.8 (J = 5.9 Hz), 96.4 (J = 5.1 Hz), 129.7–134.0, 132.5 (J = 155.3 Hz), 142.1 (J = 50.9 Hz). 31P-NMR (242.9 MHz): δ 39.7. Anal. Calcd for C26H33Br2O3P (584.32): C 53.44, H 5.69. Found: C 53.50, H 5.76.
5-Butyl-5-methyl-2,2-diphenyl-4-phenylselenenyl-3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-2,5-dihydro-1,2-oxaphosphol-2-ium chloride (7c). Orange oil, yield: 50%. Rf 0.35; IR (neat, cm−1): 1120 (C-O-C), 1444, 1487 (Ph), 1585 (C=C). 1H-NMR (600.1 MHz): δ 0.88 (t, J = 6.4 Hz, 3H, Me-CH2), 1.02–1.11, 1.24–1.36, 2.35–2.53 (overlapping multiplets, 6H, (CH2)3-Me), 1.41–1.68, 3.60–3.73, 4.79–4.84 (overlapping multiplets, 9H, OTHP), 1.67 (s, 3H, Me-C), 4.46–4.61 (m, 2H, CH2O), 7.03–8.24 (m, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 14.2, 19.4, 23.2, 23.5 (J = 4.7 Hz), 25.4, 26.0 (J = 7.8 Hz), 31.1, 38.8 (J = 7.9 Hz), 62.4, 62.9 (J = 9.7 Hz), 95.7 (J = 10.0 Hz), 98.1 (J = 4.5 Hz), 111.2–138.5, 126.2 (J = 54.3 Hz), 189.4 (J = 74.3 Hz). 31P-NMR (242.9 MHz): δ 85.7. Anal. Calcd for C32H38ClO3PSe (616.03): C 62.39, H 6.22. Found: C 62.33, H 6.15.
(1E)-3-Chloro-3-methyl-2-phenylselenenyl-1-[(tetrahydro-2H-pyran-2-yloxy)methyl]hept-1-en-1-yl diphenyl phosphine oxide (9c). Yellow oil, yield: 24%. Rf 0.61; IR (neat, cm−1): 1120 (C-O-C), 1149 (P=O), 1440, 1493 (Ph), 1614 (C=C). 1H-NMR (600.1 MHz): δ 0.86 (t, J = 6.2 Hz, 3H, Me-CH2), 1.30–1.40, 1.44–1.69, 2.01–2.21, 3.36–3.74, 4.52–4.58 (overlapping multiplets, 15H, (CH2)3-Me), OTHP), 2.17 (s, 3H, Me-C), 4.07–4.21 (m, 2H, CH2O), 7.37–7.77 (m, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 14.1, 19.3, 22.7, 25.4, 26.8, 28.8 (J = 4.8 Hz), 31.0, 42.6 (J = 4.9 Hz), 62.4, 68.7 (J = 6.0 Hz), 80.5 (J = 7.9 Hz), 96.3 (J = 5.1 Hz), 128.4 (J = 105.3 Hz), 128.5–139.2, 154.2 (J = 85.2 Hz). 31P-NMR (242.9 MHz): δ 38.7. Anal. Calcd for C32H38ClO3PSe (616.03): C 62.39, H 6.22. Found: C 62.46, H 6.26.
4-Bromo-2,2-diphenyl-3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-1-oxa-2-phosphoniaspiro[4.5]dec-3-ene bromide (7d). Orange oil, yield: 49%. Rf 0.35; IR (neat, cm−1): 1123 (C-O-C), 1435, 1490 (Ph), 1582 (C=C). 1H-NMR (600.1 MHz): δ 1.27–1.70, 1.99–2.05, 2.30–2.35, 3.60–3.77, 4.77–4.82 (overlapping multiplets, 15H, (CH2)5, OTHP), 4.38–4.50 (m, 2H, CH2O), 7.28–7.93 (m, 10H, 2Ph). 62.8, 88.6 (J = 9.9 Hz), 98.2 (J = 4.8 Hz), 110.8–133.8, 133.9 (J = 50.8 Hz), 157.7 (J = 49.0 Hz). 31P-NMR (242.9 MHz): δ 85.4. Anal. Calcd for C26H31Br2O3P (582.30): C 53.63, H 5.37. Found: C 53.70, H 5.32.
(E)-2-bromo-2-(1-bromocyclohexyl)-1-[(tetrahydro-2H-pyran-2-yloxy)methyl]vinyl diphenyl phosphine oxide (9d). Yellow oil, yield: 24%. Rf 0.62; IR (neat, cm−1): 1123 (C-O-C), 1173 (P=O), 1437, 1496 (Ph), 1620 (C=C). 1H-NMR (600.1 MHz): δ 1.26–1.37, 1.46–1.71, 2.00–2.19, 3.60–3.77, 4.54–4.59 (overlapping multiplets, 15H, (CH2)5, OTHP), 3.92–4.07 (m, 2H, CH2O), 7.51–8.10 (m, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 19.6, 22.1, 25.1, 25.5, 31.2, 39.6 (J = 5.0 Hz), 62.5, 68.2 (J = 5.8 Hz), 74.5 (J = 7.9 Hz), 96.1 (J = 5.0 Hz), 129.1–133.9, 132.2 (J = 154.7 Hz), 141.7 (J = 51.4 Hz). 31P-NMR (242.9 MHz): δ 37.2.Anal. Calcd for C26H31Br2O3P (582.30): C 53.63, H 5.37. Found: C 53.58, H 5.45.
4-Bromo-2,2-diphenyl-3-[1-(tetrahydro-2H-pyran-2-yloxy)ethyl]-1-oxa-2-phosphoniaspiro[4.5]dec-3-ene bromide (7e). Orange oil, yield: 49%. Rf 0.39; IR (neat, cm−1): 1118 (C-O-C), 1439, 1491 (Ph), 1591 (C=C). 1H-NMR (600.1 MHz): δ 1.29–1.73, 1.92–2.02, 2.27–2.33, 3.58–3.73, 4.91–4.95 (overlapping multiplets, 15H, (CH2)5, OTHP), 1.55 (d, 3H, J = 6.5 Hz, Me-CH), 4.30–4.39 (m, 1H, Me-CH), 7.31–7.89 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 19.6, 22.1 (J = 4.8 Hz), 23.7, 25.3 (J = 7.7 Hz), 25.5, 30.9, 35.8 (J = 8.0 Hz), 62.4, 76.2 (J = 5.4 Hz), 89.4 (J = 9.8 Hz), 97.4 (J = 4.8 Hz), 111.2–134.0, 134.6 (J = 51.0 Hz), 156.8 (J = 48.3 Hz). 31P-NMR (242.9 MHz): δ 83.7. Anal. Calcd for C27H33Br2O3P (596.33): C 54.38, H 5.58. Found: C 54.45, H 5.64.
(E)-2-bromo-2-(1-bromocyclohexyl)-1-[1-(tetrahydro-2H-pyran-2-yloxy)ethyl]vinyl diphenyl phosphine oxide (9e). Dark orange oil, yield: 25%. Rf 0.64; IR (neat, cm−1): 1118 (C-O-C), 1165 (P=O), 1441, 1489 (Ph), 1621 (C=C). 1H-NMR (600.1 MHz): δ 1.26–1.37, 1.40–1.71, 1.98–2.16, 3.59–3.75, 4.64–4.69 (overlapping multiplets, 15H, (CH2)5, OTHP), 1.44 (dd, 3H, J = 6.5 Hz, J = 3.4 Hz, Me-CH), 4.78–4.86 (m, 1H, Me-CH), 7.50–8.04 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 19.6, 21.8, 22.5 (J = 7.9 Hz), 25.3, 25.6, 31.3, 40.2 (J = 4.7 Hz), 62.4, 74.3 (J = 7.8 Hz), 81.4 (J = 5.0 Hz), 95.6 (J = 5.0 Hz), 129.4–134.5, 131.9 (J = 155.4 Hz), 142.3 (J = 49.7 Hz). 31P-NMR (242.9 MHz): δ 38.1. Anal. Calcd for C27H33Br2O3P (596.33): C 54.38, H 5.58. Found: C 54.32, H 5.54.
2,2-Diphenyl-4-phenylselenenyl-3-[1-(tetrahydro-2H-pyran-2-yloxy)ethyl]-1-oxa-2-phosphoniaspiro-[4.5]dec-3-ene chloride (7f). Dark orange oil, yield: 48%. Rf 0.36; IR (neat, cm−1): 1118 (C-O-C), 1436, 1488 (Ph), 1586 (C=C). 1H-NMR (600.1 MHz): δ 1.30–1.64, 1.67–1.78, 2.04–2.11, 3.58–3.74, 4.91–4.96 (overlapping multiplets, 15H, (CH2)5, OTHP), 1.48 (d, 3H, J = 6.4 Hz, Me-CH), 4.18–4.26 (m, 1H, Me-CH), 7.28–7.91 (m, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 19.5, 21.2 (J = 5.1 Hz), 23.5 (J = 7.9 Hz), 23.6, 25.6, 31.4, 34.8 (J = 7.9 Hz), 62.3, 76.8 (J = 5.3 Hz), 92.4 (J = 9.8 Hz), 97.9 (J = 4.9 Hz), 111.1–138.6, 131.4 (J = 50.7 Hz), 176.7 (J = 88.5 Hz). 31P-NMR (242.9 MHz): δ 86.5. Anal. Calcd for C33H38ClO3PSe (628.04): C 63.11, H 6.10. Found: C 63.18, H 6.16.
(E)-2-(1-chlorocyclohexyl)-2-phenylselenenyl-1-[1-(tetrahydro-2H-pyran-2-yloxy)ethyl]vinyl diphenyl phosphine oxide (9f). Light orange oil, yield: 24%. Rf 0.62; IR (neat, cm−1): 1118 (C-O-C), 1167 (P=O), 1438, 1490 (Ph), 1621 (C=C). 1H-NMR (600.1 MHz): δ 1.28–1.77, 1.99–2.18, 3.59–3.76, 4.63–4.69 (overlapping multiplets, 15H, (CH2)5, OTHP), 1.36 (dd, 3H, J = 6.5 Hz, J = 3.6 Hz, Me-CH), 4.38–4.45 (m, 1H, Me-CH), 7.37–7.74 (m, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 19.6, 20.7, 21.0 (J = 7.8 Hz), 25.6, 25.7, 31.2, 38.4 (J = 4.6 Hz), 62.3, 71.4 (J = 7.9 Hz), 81.3 (J = 4.8 Hz), 95.7 (J = 4.8 Hz), 128.4–139.4, 131.3 (J = 105.4 Hz), 152.3 (J = 71.5 Hz). 31P-NMR (242.9 MHz): δ 36.0. Anal. Calcd for C33H38ClO3PSe (628.04): C 63.11, H 6.10. Found: C 63.07, H 6.05.
5-Butyl-4-chloro-5-methyl-3-[1-methyl-1-(tetrahydro-2H-pyran-2-yloxy)ethyl]-2,2-diphenyl-2,5-dihydro-1,2-oxaphosphol-2-ium chloride (7g). Yellow oil, yield: 54%. Rf 0.38; IR (neat, cm−1): 1119 (C-O-C), 1435, 1484 (Ph), 1582 (C=C). 1H-NMR (600.1 MHz): δ 0.91 (t, J = 6.4 Hz, 3H, Me-CH2), 1.15–1.21, 1.27–1.34, 2.28–2.41 (overlapping multiplets, 6H, (CH2)3-Me), 1.42–1.65, 3.69–3.84, 4.97–5.02 (overlapping multiplets, 9H, OTHP), 1.68 (s, 3H, Me-C), 1.70 (s, 6H, Me2C), 7.63–8.26 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 14.1, 20.5, 23.2, 23.8 (J = 4.5 Hz), 25.3, 26.1 (J = 7.8 Hz), 29.7 (J = 8.1 Hz), 32.3, 39.1 (J = 7.8 Hz), 63.7, 79.5 (J = 9.8 Hz), 92.7 (J = 9.7 Hz), 95.7 (J = 4.7 Hz), 106.5–134.6, 133.4 (J = 50.2 Hz), 164.8 (J = 40.5 Hz). 31P-NMR (242.9 MHz): δ 82.0. Anal. Calcd for C28H37Cl2O3P (523.47): C 64.24, H 7.12. Found: C 64.19, H 7.05.
(1E)-2,3-dichloro-3-methyl-1-[1-methyl-1-(tetrahydro-2H-pyran-2-yloxy)ethyl]hept-1-en-1-yl diphenyl phosphine oxide (9g). Orange oil, yield: 25%. Rf 0.63; IR (neat, cm−1): 1119 (C-O-C), 1159 (P=O), 1439, 1485 (Ph), 1617 (C=C). 1H-NMR (600.1 MHz): δ 0.87 (t, J = 6.3 Hz, 3H, Me-CH2), 1.35–1.48, 1.54–1.69, 2.27–2.54 (overlapping multiplets, 6H, (CH2)3-Me), 1.47–1.68, 3.66–3.80, 4.70–4.76 (overlapping multiplets, 9H, OTHP), 1.58 (s, 3H, Me2C), 1.83 (s, 3H, Me-C), 7.53–7.91 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 14.0, 20.6, 22.9, 25.2, 26.4, 29.5 (J = 4.7 Hz), 30.8 (J = 7.9 Hz), 32.2, 42.9 (J = 4.7 Hz), 63.6, 76.4 (J = 7.8 Hz), 80.3 (J = 9.9 Hz), 93.4 (J = 4.7 Hz), 129.3–134.2, 133.6 (J = 101.4 Hz), 152.9 (J = 41.2 Hz). 31P-NMR (242.9 MHz): δ 37.7. Anal. Calcd for C28H37Cl2O3P (523.47): C 64.24, H 7.12. Found: C 64.28, H 7.20.
3.6. General Procedure for the Reactions of the 2-Diphenylphosphinoyl-2,3-dien-1-ols 4a–c and 3-Diphenylphosphinoyl-3,4-dien-2-ols 4d,e with Electrophilic Reagents
To a solution of the 2-diphenylphosphinoyl-2,3-dien-1-ols
4a–
c or the 3-diphenylphosphinoyl-3,4-dien-2-ols
4d,
e (3.0 mmol) in dry dichloromethane(10 mL) at −20 °C was added dropwise with stirring a solution of electrophilic reagent (sulfuryl chloride, bromine, benzenesulfenyl chloride, benzeneselenenyl chloride) (3.6 mmol) in the same solvent (10 mL). The reaction mixture was stirred at the same temperature for several hours (see
Table 2) and an hour at room temperature. The solvent was removed using a rotatory evaporator and the residue was purified by column chromatography (silica gel, ethyl acetate and hexane 2:1). The pure products
8 and
10 had the following properties:
4-Bromo-5-ethyl-3-(hydroxymethyl)-5-methyl-2,2-diphenyl-2,5-dihydro-1,2-oxaphosphol-2-ium bromide (8a). Pale orange oil, yield: 52%. Rf 0.38; IR (neat, cm−1): 1435, 1485 (Ph), 1581 (C=C), 3375 (OH). 1H-NMR (600.1 MHz): δ 1.08 (t, J = 7.3 Hz, 3H, Me-CH2), 1.80 (s, 3H, Me-C), 2.31–2.40 (m, 2H, Me-CH2), 4.57 (s, 1H, OH), 4.95–5.02 (m, 2H, CH2O), 7.80–8.45 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 8.1 (J = 4.6 Hz), 27.2 (J = 7.8 Hz), 31.0 (J = 7.7 Hz), 60.2 (J = 5.8 Hz), 92.7 (J = 9.7 Hz), 111.5–135.1, 133.7 (J = 49.7 Hz), 158.1 (J = 50.3 Hz). 31P-NMR (242.9 MHz): δ 88.1. Anal. Calcd for C19H21Br2O2P (472.15): C 48.33, H 4.48. Found: C 48.26, H 4.55.
(2E)-3,4-Dibromo-2-diphenylphosphinoyl-4-methylhex-2-en-1-ol (10a). Yellow oil, yield: 23%. Rf 0.64; IR (neat, cm−1): 1171 (P=O), 1433, 1482 (Ph), 1628 (C=C), 3374 (OH). 1H-NMR (600.1 MHz): δ 1.14 (t, J = 7.3 Hz, 3H, Me-CH2), 1.98–2.18 (m, 2H, Me-CH2), 2.18 (s, 3H, Me-C), 2.98 (s, 1H, OH), 4.53–4.57 (m, 2H, CH2O), 7.53–8.08 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 9.2, 35.2 (J = 5.0 Hz), 36.4 (J = 5.0 Hz), 62.0 (J = 7.9 Hz), 63.6 (J = 5.9 Hz), 129.4–133.7, 131.5 (J = 49.9 Hz), 141.7 (J = 50.8 Hz). 31P-NMR (242.9 MHz): δ 39.6. Anal. Calcd for C19H21Br2O2P (472.15): C 48.33, H 4.48. Found: C 48.40, H 4.52.
5-Butyl-3-(hydroxymethyl)-5-methyl-2,2-diphenyl-4-phenylselenenyl-2,5-dihydro-1,2-oxaphosphol-2-ium chloride (8b). Yellow oil, yield: 46%. Rf 0.39; IR (neat, cm−1): 1438, 1487 (Ph), 1585 (C=C), 3380 (OH). 1H-NMR (600.1 MHz): δ 0.92 (t, J = 7.2 Hz, 3H, Me-CH2), 1.02–1.11, 1.27–1.37, 2.35–2.54 (m, 6H, (CH2)3-Me), 1.67 (s, 3H, Me-C), 5.08–5.13 (m, 2H, CH2O), 5.24 (s, 1H, OH), 6.99–8.28 (overlapping multiplets, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 13.8, 22.1 (J = 5.1 Hz), 23.4, 26.7 (J = 7.9 Hz), 38.5 (J = 7.9 Hz), 60.5 (J = 5.8 Hz), 95.8 (J = 9.9 Hz), 111.4–138.6, 126.2 (J = 51.3 Hz), 189.0 (J = 69.4 Hz). 31P-NMR (242.9 MHz): δ 86.9. Anal. Calcd for C27H30ClO2PSe (531.91): C 60.97, H 5.68. Found: C 60.92, H 5.73.
(2E)-4-Chloro-2-diphenylphosphinoyl-4-methyl-3-phenylselenenyl-oct-2-en-1-ol (10b). Yellow oil, yield: 26%. Rf 0.65; IR (neat, cm−1): 1175 (P=O), 1437, 1490 (Ph), 1620 (C=C), 3389 (OH). 1H-NMR (600.1 MHz): δ 0.88 (t, J = 7.1 Hz, 3H, Me-CH2), 1.30–1.46, 2.36–2.63 (m, 6H, (CH2)3-Me), 1.79 (s, 3H, Me-C), 3.74 (s, 1H, OH), 4.67–4.72 (m, 2H, CH2O), 7.35–7.98 (overlapping multiplets, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 14.1, 22.9, 25.7, 28.9 (J = 4.8 Hz), 42.4 (J = 4.7 Hz), 64.7 (J = 6.0 Hz), 80.0 (J = 7.9 Hz), 127.7 (J = 101.4 Hz), 129.0–139.1, 153.6 (J = 57.4 Hz). 31P-NMR (242.9 MHz): δ 39.9. Anal. Calcd for C27H30ClO2PSe (531.91): C 60.97, H 5.68. Found: C 61.02, H 5.75.
4-Chloro-3-(hydroxymethyl)-2,2-diphenyl-1-oxa-2-phosphonia-spiro[4.5]dec-3-ene chloride (8c). Yellow oil, yield: 54%. Rf 0.37; IR (neat, cm−1): 1441, 1489 (Ph), 1582 (C=C), 3389 (OH). 1H-NMR (600.1 MHz): δ 1.30–1.49, 1.61–1.88, 2.18–2.24 (overlapping multiplets, 10H, (CH2)5), 4.99–5.04 (m, 2H, CH2O), 5.30 (s, 1H, OH), 7.75–8.31 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 22.5 (J = 4.6 Hz), 24.0, 35.6 (J = 7.8 Hz), 60.3 (J = 5.8 Hz), 90.3 (J = 9.8 Hz), 106.3–133.8, 127.1 (J = 49.7 Hz), 171.4 (J = 42.6 Hz). 31P-NMR (242.9 MHz): δ 86.5. Anal. Calcd for C21H23Cl2O2P (409.29): C 61.63, H 5.66. Found: C 61.70, H 5.71.
(2E)-3-Chloro-3-(1-chlorocyclohexyl)-2-diphenylphosphinoyl-prop-2-en-1-ol (10c). Pale orange oil, yield: 26%. Rf 0.62; IR (neat, cm−1): 1178 (P=O), 1440, 1493 (Ph), 1619 (C=C), 3384 (OH). 1H-NMR (600.1 MHz): δ 1.28–1.41, 1.55–1.70, 1.76–1.92 (overlapping multiplets, 10H, (CH2)5), 3.77 (s, 1H, OH), 4.58–4.63 (m, 2H, CH2O), 7.43–7.99 (m, 10H, 2Ph). 13C-NMR (150.9 MHz) δ = 21.8, 25.7, 38.5 (J = 5.1 Hz), 63.7 (J = 5.9 Hz), 68.3 (J = 9.9 Hz), 129.1 (J = 100.9 Hz), 129.5–134.4, 148.7 (J = 39.7 Hz). 31P-NMR (242.9 MHz): δ 34.6. Anal. Calcd for C21H23Cl2O2P (409.29): C 61.63, H 5.66. Found: C 61.69, H 5.60.
3-(1-Hydroxyethyl)-2,2-diphenyl-4-phenylsulfenyl-1-oxa-2-phosphonia-spiro[4.5]dec-3-ene chloride (8d). Yellow oil, yield: 45%. Rf 0.38; IR (neat, cm−1): 1435, 1494 (Ph), 1580 (C=C), 3393 (OH). 1H-NMR (600.1 MHz): δ 1.31–1.47, 1.71–1.97, 2.07–2.13 (overlapping multiplets, 10H, (CH2)5), 1.78 (dd, J = 16.6 Hz,J = 6.6 Hz, 3H, Me-CH), 4.32 (s, 1H, OH), 4.96–5.07 (m, 1H, Me-CH), 6.91–8.60 (overlapping multiplets, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 22.8 (J = 5.1 Hz), 23.6, 26.2 (J = 7.9 Hz), 35.8 (J = 7.8 Hz), 70.6 (J = 5.1 Hz), 89.9 (J = 9.9 Hz), 125.6–139.1, 133.4 (J = 51.0 Hz), 164.7 (J = 15.1 Hz). 31P-NMR (242.9 MHz): δ 86.0. Anal. Calcd for C28H30ClO2PS (497.03): C 67.66, H 6.08. Found: C 67.71, H 6.12.
(3E)-4-(1-Chlorocyclohexyl)-3-diphenylphosphinoyl-4-phenylsulfenyl-but-3-en-2-ol (10d). Orange oil, yield: 25%. Rf 0.62; IR (neat, cm−1): 1169 (P=O), 1441, 1488 (Ph), 1618 (C=C), 3391 (OH). 1H-NMR (600.1 MHz): δ 1.34–1.46, 1.49–1.54, 1.59–1.78 (overlapping multiplets, 10H, (CH2)5), 1.34 (dd, J = 15.3 Hz,J = 6.5 Hz, 3H, Me-CH), 3.88 (s, 1H, OH), 4.63–4.74 (m, 1H, Me-CH), 7.36–7.71 (overlapping multiplets, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 22.7, 23.4 (J = 7.9 Hz), 25.6, 38.9 (J = 4.6 Hz), 68.1 (J = 7.9 Hz), 76.3 (J = 5.0 Hz), 126.5–137.4, 133.2 (J = 101.0 Hz), 162.4 (J = 15.0 Hz). 31P-NMR (242.9 MHz): δ 34.0.Anal. Calcd for C28H30ClO2PS (497.03): C 67.66, H 6.08. Found: C 67.59, H 6.13.
5-Butyl-3-(1-hydroxy-1-methylethyl)-5-methyl-2,2-diphenyl-4-phenylselenenyl-2,5-dihydro-1,2-oxa-phosphol-2-ium chloride (8e). Yellow oil, yield: 46%. Rf 0.40; IR (neat, cm−1): 1438, 1487 (Ph), 1585 (C=C), 3393 (OH). 1H-NMR (600.1 MHz): δ 0.88 (t, J = 7.1 Hz, 3H, Me-CH2), 1.03–1.11, 1.27–1.34, 2.36–2.50 (m, 6H, (CH2)3-Me), 1.58 (s, 3H, Me2C), 1.64 (s, 3H, Me-C), 5.24 (s, 1H, OH), 6.94–8.14 (overlapping multiplets, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 14.1, 22.1 (J = 5.1 Hz), 23.4, 26.4 (J = 7.8 Hz), 32.7 (J = 8.0 Hz), 38.4 (J = 7.8 Hz), 81.4 (J = 9.7 Hz), 97.4 (J = 9.8 Hz), 112.7–138.4, 135.3 (J = 53.7 Hz), 178.0 (J = 69.4 Hz). 31P-NMR (242.9 MHz): δ 89.4. Anal. Calcd for C29H34ClO2PSe (559.97): C 62.20, H 6.12. Found: C 62.26, H 6.05.
(3E)-5-Chloro-3-diphenylphosphinoyl-2,5-dimethyl-4-phenylselenenyl-non-3-en-2-ol (10e). Yellow oil, yield: 24%. Rf 0.65; IR (neat, cm−1): 1170 (P=O), 1438, 1486 (Ph), 1625 (C=C), 3396 (OH). 1H-NMR (600.1 MHz): δ 0.89 (t, J = 7.3 Hz, 3H, Me-CH2), 1.30–1.44, 2.38–2.61 (m, 6H, (CH2)3-Me), 1.46, 1.48 (ss, 3H, Me2C), 1.77 (s, 3H, Me-C), 4.13 (s, 1H, OH), 7.38–7.69 (overlapping multiplets, 15H, 3Ph). 13C-NMR (150.9 MHz) δ = 14.1, 22.9, 25.48, 29.3 (J = 5.1 Hz), 29.9 (J = 7.8 Hz), 43.3 (J = 4.8 Hz), 82.3 (J = 7.8 Hz), 82.8 (J = 9.9 Hz), 128.4–139.1, 135.8 (J = 102.7 Hz), 153.7 (J = 15.1 Hz). 31P-NMR (242.9 MHz): δ 36.9. Anal. Calcd for C29H34ClO2PSe (559.97): C 62.20, H 6.12. Found: C 62.25, H 6.08.