Active Components with Inhibitory Activities on IFN-γ/STAT1 and IL-6/STAT3 Signaling Pathways from Caulis Trachelospermi
Abstract
:1. Introduction

2. Results and Discussion
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 128.8 | 133.0 | ||
| 2 | 106.0 | 6.34 (1H, s) | 113.0 | 6.70 (1H, d, 1.6) |
| 3 | 148.0 | 148.8 | ||
| 4 | 133.9 | 145.1 | ||
| 5 | 148.0 | 115.3 | 6.97 (1H, d, 8.3) | |
| 6 | 106.0 | 6.34 (1H, s) | 120.5 | 6.61 (1H, br d, 8.3) |
| 7 | 37.3 | 2.45–2.52 (2H, m) | 30.9 | 2.61 (1H, dd, 12.3, 2.4) |
| 2.44 (1H, br d, 12.3) | ||||
| 8 | 40.9 | 2.40–2.45 (1H, m) | 42.8 | 2.39 (1H, m) |
| 9 | 70.8 | 4.11 (1H, dd, 8.5, 7.2) | 70.0 | 3.95 (2H, d, 7.7) |
| 3.89 (1H, t, 8.5) | ||||
| 1' | 131.9 | 126.4 | ||
| 2' | 113.9 | 6.80 (1H, d, 1.8) | 114.5 | 6.77 (1H, br s) |
| 3' | 148.7 | 147.2 | ||
| 4' | 145.4 | 145.4 | ||
| 5' | 115.1 | 6.99 (1H, d, 8.3) | 115.3 | 6.68 (1H, d, 8.0) |
| 6' | 121.4 | 6.68 (1H, dd, 8.3, 1.8) | 122.7 | 6.61 (1H, br d, 8.0) |
| 7' | 33.6 | 2.80–2.83 (2H, m) | 40.0 (overlapped) | 2.98 (1H, d, 13.8) |
| 2.83 (1H, d, 13.8) | ||||
| 8' | 45.6 | 2.75 (1H, dd, 8.2, 6.1) | 75.4 | |
| 9' | 178.6 | 178.1 | ||
| 1'' | 100.3 | 4.84 (1H, d, 7.3) | 100.2 | 4.82 (1H, d, 7.2) |
| 2'' | 73.3 | 73.2 | ||
| 3'' | 77.0 | 77.0 | ||
| 4'' | 69.7 | 69.7 | ||
| 5'' | 76.9 | 76.9 | ||
| 6'' | 60.7 | 60.7 | ||
| 4-OH | 8.17 (1H, s) | |||
| 4'-OH | 8.85 (1H, s) | |||
| 8'-OH | 6.21 (1H, s) | |||
| 3-OCH3 | 56.0 | 3.71 (3H, s) | 55.6 | 3.70 (3H, s) |
| 3'-OCH3 | 55.7 | 3.72 (3H, s) | 55.6 | 3.73 (3H, s) |
| 5-OCH3 | 56.0 | 3.71 (3H, s) | ||

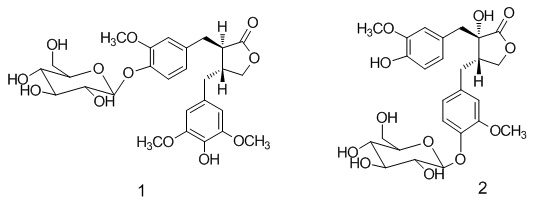
 −48.5 (c 0.19, MeOH) revealed its absolute configuration was 8S, 8'S. Compound 2 was accordingly assigned as nortrachelogenin 4-O-β-d-glucopyranoside, structurally (8S,8'S)-4',8'-dihydroxy-3,3'-dimethoxylignan-9,9'-olide-4-O-β-d-glucopyranoside. A publication of patent application has concerned the same planar structure of 2 by covering with common structures [20], but no data of the production (isolation or synthesis) and structure elucidation was provided. Therefore, we report herein 2 as a new compound.
−48.5 (c 0.19, MeOH) revealed its absolute configuration was 8S, 8'S. Compound 2 was accordingly assigned as nortrachelogenin 4-O-β-d-glucopyranoside, structurally (8S,8'S)-4',8'-dihydroxy-3,3'-dimethoxylignan-9,9'-olide-4-O-β-d-glucopyranoside. A publication of patent application has concerned the same planar structure of 2 by covering with common structures [20], but no data of the production (isolation or synthesis) and structure elucidation was provided. Therefore, we report herein 2 as a new compound.| Sample | IFN-γ/STAT1 | IL-6/STAT3 |
|---|---|---|
| extract | 2.43 μg/mL | 1.38 μg/mL |
| trachelogenin | 3.14 μM | 3.63 μM |
| arctigenin | 9.46 μM | 6.47 μM |
| matairesinol | - | 2.92 μM |
| Pyridone 6 a | 0.0049 μM | - |
| AG490 a | - | >100 μM |
| Compound | Conc. (μM) | IFN-γ/STAT1 | IL-6/STAT3 |
|---|---|---|---|
| trachelogenin | 5 | 77.5% | 96.1% |
| tracheloside | 5 | 9.4% | 13.5% |
| trachelogenin 4'- O-β-gentiobioside | 5 | −8.0% | −17.5% |
| nortrachelogenin | 5 | −28.9% | 27.3% |
| nortracheloside | 5 | −4.2% | 2.3% |
| nortrachelogenin 8'- O-β-d-glucoside | 5 | −7.4% | 1.6% |
| nortrachelogenin 5'- C-β-d-glucoside | 5 | −22.1% | −9.3% |
| nortrachelogenin 4- O-β-d-glucopyranoside (2) | 5 | −3.5% | 10.9% |
| nortrachelogenin 4,4'-di- O-β-d-glucopyranoside (4) | 5 | −2.7% | −14.7% |
| arctigenin | 5 | 32.6% | 44.2% |
| arctiin | 5 | 15.4% | −14.7% |
| matairesinol | 5 | 7.2% | 89.8% |
| matairesinoside | 5 | −9.4% | 13.5% |
| matairesinol 4- O-β-d-glucopyranoside (3) | 5 | 5.8% | 7.0% |
| matairesinol 4'- O-β-gentiobioside | 5 | −12.0% | −12.9% |
| traxillagenin | 5 | −1.9% | −26.1% |
| traxillaside | 5 | −5.1% | −19.8% |
| 4-demethyltraxillagenin | 5 | 7.1% | 22.4% |
| 4-demethyltraxillaside (1) | 5 | 6.3% | −10.5% |
| 5-methoxytrachelogenin | 5 | −0.2% | 14.3% |
| 5-methoxytracheloside | 5 | −21.0% | 4.8% |
3. Experimental Section
3.1. General
3.2. Plant and Compound Materials
3.3. Extraction and Isolation
3.4. Spectral Data
 −44.1 (c 0.52, MeOH). CD (MeOH) λmax nm (∆ε): 233 (−2.72), 276 (−0.58). ESIMS: m/z 573 [M+Na]+ (Pos.), 549 [M−H]− (Neg.). HRESIMS: m/z: 573.1945 [M+Na]+ (Calcd. for C27H34O12Na, 573.1942). 1H-NMR and 13C-NMR data see Table 1.
−44.1 (c 0.52, MeOH). CD (MeOH) λmax nm (∆ε): 233 (−2.72), 276 (−0.58). ESIMS: m/z 573 [M+Na]+ (Pos.), 549 [M−H]− (Neg.). HRESIMS: m/z: 573.1945 [M+Na]+ (Calcd. for C27H34O12Na, 573.1942). 1H-NMR and 13C-NMR data see Table 1. −48.5 (c 0.19, MeOH). CD (MeOH) λmax nm (∆ε): 233 (−1.94), 276 (−0.28). ESIMS: m/z 559 [M+Na]+ (Pos.), 535 [M−H]−(Neg.). HRESIMS: m/z: 559.1788 [M+Na]+ (Calcd. for C26H32O12Na, 559.1786). 1H-NMR and 13C-NMR data see Table 1.
−48.5 (c 0.19, MeOH). CD (MeOH) λmax nm (∆ε): 233 (−1.94), 276 (−0.28). ESIMS: m/z 559 [M+Na]+ (Pos.), 535 [M−H]−(Neg.). HRESIMS: m/z: 559.1788 [M+Na]+ (Calcd. for C26H32O12Na, 559.1786). 1H-NMR and 13C-NMR data see Table 1. −34.0 (c 0.40, MeOH). CD (MeOH) λmax nm (∆ε): 233 (−2.23), 276 (−0.39). ESIMS: m/z 543 [M+Na]+ (Pos.), 519 [M−H]−(Neg.). HRESIMS: m/z: 543.1838 [M+Na]+ (Calcd. for C26H32O11Na, 543.1837). 1H-NMR (DMSO-d6): δH 6.67 (1H, d, J = 1.8 Hz, H-2), 6.96 (1H, d, J = 8.3 Hz, H-5), 6.57 (1H, dd, J = 8.3, 1.8 Hz, H-6), 2.44–2.48 (3H, m, H-7, 8), 4.05 (1H, m, Ha-9), 3.87 (1H, dd, J = 11.2, 4.8 Hz, Hb-9), 6.76 (1H, d, J = 1.8 Hz, H-2'), 6.69 (1H, d, J = 8.0 Hz, H-5'), 6.60 (1H, dd, J = 8.0, 1.8 Hz, H-6'), 2.83 (1H, dd, J = 13.5, 5.1 Hz, Ha-7'), 2.73 (1H, m, Hb-7'), 2.69 (1H, m, H-8'), 8.84 (1H, s, 4'-OH), 3.72 (6H, s, 3,3'-OMe), 4.82 (1H, d, J = 7.4 Hz, H-1''). 13C-NMR (DMSO-d6): δc 132.6 (C-1), 112.9 (C-2), 148.8 (C-3), 145.1 (C-4), 115.3 (C-5), 120.5 (C-6), 36.9 (C-7), 40.9 (C-8), 70.8 (C-9), 129.0 (C-1'), 113.5 (C-2'), 147.5 (C-3'), 145.1 (C-4'), 115.4 (C-5'), 121.6 (C-6'), 33.8 (C-7'), 45.7 (C-8'), 178.6 (C-9'), 100.2 (C-1''), 73.3 (C-2''), 77.1 (C-3''), 69.7 (C-4''), 76.9 (C-5''), 60.7 (C-6''), 55.6 (C-3, 3'-OMe).
−34.0 (c 0.40, MeOH). CD (MeOH) λmax nm (∆ε): 233 (−2.23), 276 (−0.39). ESIMS: m/z 543 [M+Na]+ (Pos.), 519 [M−H]−(Neg.). HRESIMS: m/z: 543.1838 [M+Na]+ (Calcd. for C26H32O11Na, 543.1837). 1H-NMR (DMSO-d6): δH 6.67 (1H, d, J = 1.8 Hz, H-2), 6.96 (1H, d, J = 8.3 Hz, H-5), 6.57 (1H, dd, J = 8.3, 1.8 Hz, H-6), 2.44–2.48 (3H, m, H-7, 8), 4.05 (1H, m, Ha-9), 3.87 (1H, dd, J = 11.2, 4.8 Hz, Hb-9), 6.76 (1H, d, J = 1.8 Hz, H-2'), 6.69 (1H, d, J = 8.0 Hz, H-5'), 6.60 (1H, dd, J = 8.0, 1.8 Hz, H-6'), 2.83 (1H, dd, J = 13.5, 5.1 Hz, Ha-7'), 2.73 (1H, m, Hb-7'), 2.69 (1H, m, H-8'), 8.84 (1H, s, 4'-OH), 3.72 (6H, s, 3,3'-OMe), 4.82 (1H, d, J = 7.4 Hz, H-1''). 13C-NMR (DMSO-d6): δc 132.6 (C-1), 112.9 (C-2), 148.8 (C-3), 145.1 (C-4), 115.3 (C-5), 120.5 (C-6), 36.9 (C-7), 40.9 (C-8), 70.8 (C-9), 129.0 (C-1'), 113.5 (C-2'), 147.5 (C-3'), 145.1 (C-4'), 115.4 (C-5'), 121.6 (C-6'), 33.8 (C-7'), 45.7 (C-8'), 178.6 (C-9'), 100.2 (C-1''), 73.3 (C-2''), 77.1 (C-3''), 69.7 (C-4''), 76.9 (C-5''), 60.7 (C-6''), 55.6 (C-3, 3'-OMe).3.5. Cell Lines and Reagents
3.6. Luciferase Assay
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Committee of Pharmacopoeia of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China (First Part); China Medical Science Technology Press: Beijing, China, 2010; p. 252. [Google Scholar]
- Nishibe, S.; Han, Y.M. Chemical constituents from Trachelosperomum jasminoides and its anticancer activity. World Phytomed. 2002, 17, 57–58. [Google Scholar]
- Lee, M.H.; Lee, J.M.; Jun, S.H.; Ha, C.G.; Lee, S.-H.; Kim, N.M.; Lee, J.H.; Ko, N.Y.; Mun, S.H.; Park, S.H.; et al. In vitro and in vivo anti-inflammatory action of the ethanol extract of Trachelospermi caulis. J. Pharm. Pharmacol. 2007, 59, 123–130. [Google Scholar] [CrossRef]
- Tan, X.Q.; Chen, H.S.; Liu, R.H.; Tan, C.H.; Xu, C.L.; Xuan, W.D.; Zhang, W.D. Lignans from Trachelospermum jasminoides. Planta Med. 2005, 71, 93–95. [Google Scholar] [CrossRef]
- Tan, X.Q.; Chen, H.S.; Zhou, M.; Zhang, Y. Triterpenoids from canes with leaves of Trachelospermum jasminoides. Chin. Tradit. Herb. Drugs 2006, 37, 171–174. [Google Scholar]
- Tan, X.Q.; Guo, L.J.; Chen, H.S.; Wu, L.S.; Kong, F.F. Study on the flavonoids constituents of Trachelospermum jasminoides. J. Chin. Med. Mater. 2010, 33, 58–60. [Google Scholar]
- Yu, N.J.; Zhao, Y.M.; Ren, F.X. Total Lignans Extract from Caulis Trachelospermi, Its Extraction Method, and the Medicinal Usage of the Extract and Its Active Constituents. CN 200510093357.X, 26 August 2005. [Google Scholar]
- Zhu, C.C.; Jing, L.; Yu, N.J.; Yang, X.D.; Zhao, Y.M. A new lignan and active compounds inhibiting NF-κB signaling pathway from Caulis Trachelospermi. Acta Pharm. Sin. B 2013, 3, 109–112. [Google Scholar]
- Jing, L.; Yu, N.J.; Zhao, Y.M.; Li, Y.S. Trace chemical constituents contained in Trachelospermum jasminoides and structure identification. China J. Chin. Mater. Med. 2012, 37, 1581–1585. [Google Scholar]
- Yuan, Q.S.; Yu, N.J.; Zhao, Y.M.; Xu, B.; Yao, Z.W. Chemical constituents from Trachelospermum jasminoides. Chin. Tradit. Herb. Drugs 2010, 41, 179–181. [Google Scholar]
- Jing, L.; Yu, N.J.; Li, Y.S.; Fu, L.; Zhao, Y.M. Novel lignans from the stems and leaves of Trachelospermum jasminoides. Chin. Chem. Lett. 2011, 22, 1075–1077. [Google Scholar] [CrossRef]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002, 8, 945–954. [Google Scholar]
- Al Siddiquee, Z.K.; Turkson, J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008, 18, 254–267. [Google Scholar]
- De Hooge, A.S.; van de Loo, F.A.; Koenders, M.I.; Bennink, M.B.; Arntz, Q.J.; Kolbe, T.; van den Berg, W.B. Local activation of STAT-1 and STAT-3 in the inflamed synovium during zymosan-induced arthritis. Arthritis Rheum. 2004, 50, 2014–2023. [Google Scholar]
- Liu, Y.Q.; Yu, N.J.; Yang, X.D.; Zhao, Y.M. Study on HPLC fingerprint of Trachelospermum jasminoides. China J. Chin. Mater. Med. 2009, 34, 727–730. [Google Scholar]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar]
- Gu, L.H.; Wang, S.X.; Li, X.; Zhu, T.R. Studies on antibacterial constituents from Gerbenra anandria (L.) Sch. Bip. Acta Pharmacol. Sin. 1987, 22, 272–277. [Google Scholar]
- Khamlach, K.; Dhal, R.; Brown, E. Total syntheses of (−)-trachelogenin, (−)-nortrachelogenin and (+)-wikstromol. Tetrahedron Lett. 1989, 30, 2221–2224. [Google Scholar]
- Peuhu, E.; Eriksson, J.; Holmbom, T.; Eklund, P.; Sjoholm, R. Pharmaceutical compositions comprising 8-substituted dibenzylbutyrolactone lignans. U.S. Patent 2013/0281381 A1, 24 October 2013. [Google Scholar]
- Palter, R.; Lundin, R.E. A bitter principle of safflower; Matairesinol monoglucoside. Phytochemistry 1970, 9, 2407–2409. [Google Scholar] [CrossRef]
- Palter, R.; Haddon, W.F.; Lundin, R.E. The complete structure of matairesinol monoglugoside. Phytochemistry 1971, 10, 1587–1589. [Google Scholar]
- Nishibe, S.; Hisada, S.; Inagaki, I. Lignan diglucosides from Trachelospermum asiaticum. Phytochemistry 1972, 11, 3084–3085. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. Lignans of Trachelospermum asiaticum var. intermedium. V. Isolation of nortrachelogenin-4,4'-di-O-β-d-glucopyranoside. Chem. Pharm. Bull. 1973, 21, 1114–1117. [Google Scholar] [CrossRef]
- Steinbeck, C.; Schneider, C.; Rotscheidt, K.; Breitmaier, E. A 4-methy-7-hydroxyphthalide glycoside and other constituents from Quillaja saponaria molina. Phytochemistry 1995, 40, 1313–1315. [Google Scholar] [CrossRef]
- Verotta, L.; Dell’Agli, M.; Giolito, A.; Guerrini, M.; Cabalion, P.; Bosisio, E. In vitro antiplasmodial activity of extracts of Tristaniopsis species and identification of the active constituents: Ellagic acid and 3,4,5-trimethoxyphenyl-(6'-O-galloyl)-O-β-d-glucopyranoside. J. Nat. Prod. 2001, 64, 603–607. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic glycosides from Morinda coreia. Phytochemistry 2002, 59, 551–556. [Google Scholar] [CrossRef]
- Zhang, J.M.; Shi, X.F.; Ma, Q.H.; He, F.J.; Fan, B.; Wang, D.D.; Liu, D.Y. Chemical constituents from pine needles of Cedrus deodara. Chem. Nat. Compd. 2011, 47, 272–274. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Tanegoside A, B and C, lignan glycosides from Trachelospermum liukiuense. Chem. Pharm. Bull. 1990, 38, 2143–2145. [Google Scholar]
- Li, L.; Meng, F.H.; Guo, J.F.; Sun, L.; Yu, N.J.; Zhao, Y.M. Simultaneous quantification of tracheloside and trachelogenin in rat plasma using liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 1033–1037. [Google Scholar]
- Nose, M.; Fujimoto, T.; Takeda, T.; Nishibe, S.; Ogihara, Y. Structural transformation of lignan compounds in rat gastrointestinal tract. Planta Med. 1992, 58, 520–523. [Google Scholar]
- Wang, Y.; Ma, X.Q.; Yan, S.S.; Shen, S.S.; Zhu, H.L.; Gu, Y.; Wang, H.B.; Qin, G.W.; Yu, Q. 17-Hydroxy-jolkinolide B inhibits signal transducers and activators of transcription 3 signaling by covalently cross-linking Janus Kinases and induces apoptosis of human cancer cells. Cancer Res. 2009, 69, 7302–7310. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.-T.; Wang, Z.-X.; Yang, Y.; Wang, L.; Sun, R.-F.; Zhao, Y.-M.; Yu, N.-J. Active Components with Inhibitory Activities on IFN-γ/STAT1 and IL-6/STAT3 Signaling Pathways from Caulis Trachelospermi. Molecules 2014, 19, 11560-11571. https://doi.org/10.3390/molecules190811560
Liu X-T, Wang Z-X, Yang Y, Wang L, Sun R-F, Zhao Y-M, Yu N-J. Active Components with Inhibitory Activities on IFN-γ/STAT1 and IL-6/STAT3 Signaling Pathways from Caulis Trachelospermi. Molecules. 2014; 19(8):11560-11571. https://doi.org/10.3390/molecules190811560
Chicago/Turabian StyleLiu, Xiao-Ting, Zhe-Xing Wang, Yu Yang, Lin Wang, Ruo-Feng Sun, Yi-Min Zhao, and Neng-Jiang Yu. 2014. "Active Components with Inhibitory Activities on IFN-γ/STAT1 and IL-6/STAT3 Signaling Pathways from Caulis Trachelospermi" Molecules 19, no. 8: 11560-11571. https://doi.org/10.3390/molecules190811560