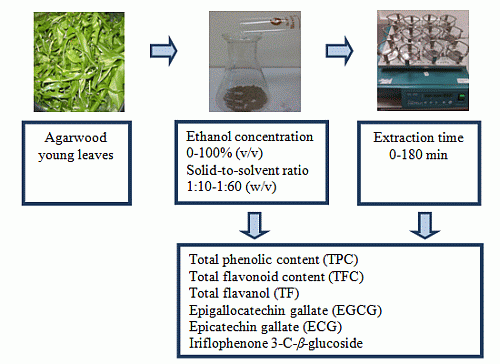
Assessment of Extraction Parameters on Antioxidant Capacity, Polyphenol Content, Epigallocatechin Gallate (EGCG), Epicatechin Gallate (ECG) and Iriflophenone 3-C-β-Glucoside of Agarwood (Aquilaria crassna) Young Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ethanol Concentration Evaluation
2.1.1. Polyphenol Content of the CPE of Aquilaria crassna

2.1.2. Antioxidant Capacity of the CPE of Aquilaria crassna
2.1.3. Quantitative and Qualitative Investigation of the CPE of Aquilaria crassna
2.2. Solid-to-Solvent Ratio Evaluation
2.2.1. Polyphenol Content of the CPE of Aquilaria crassna
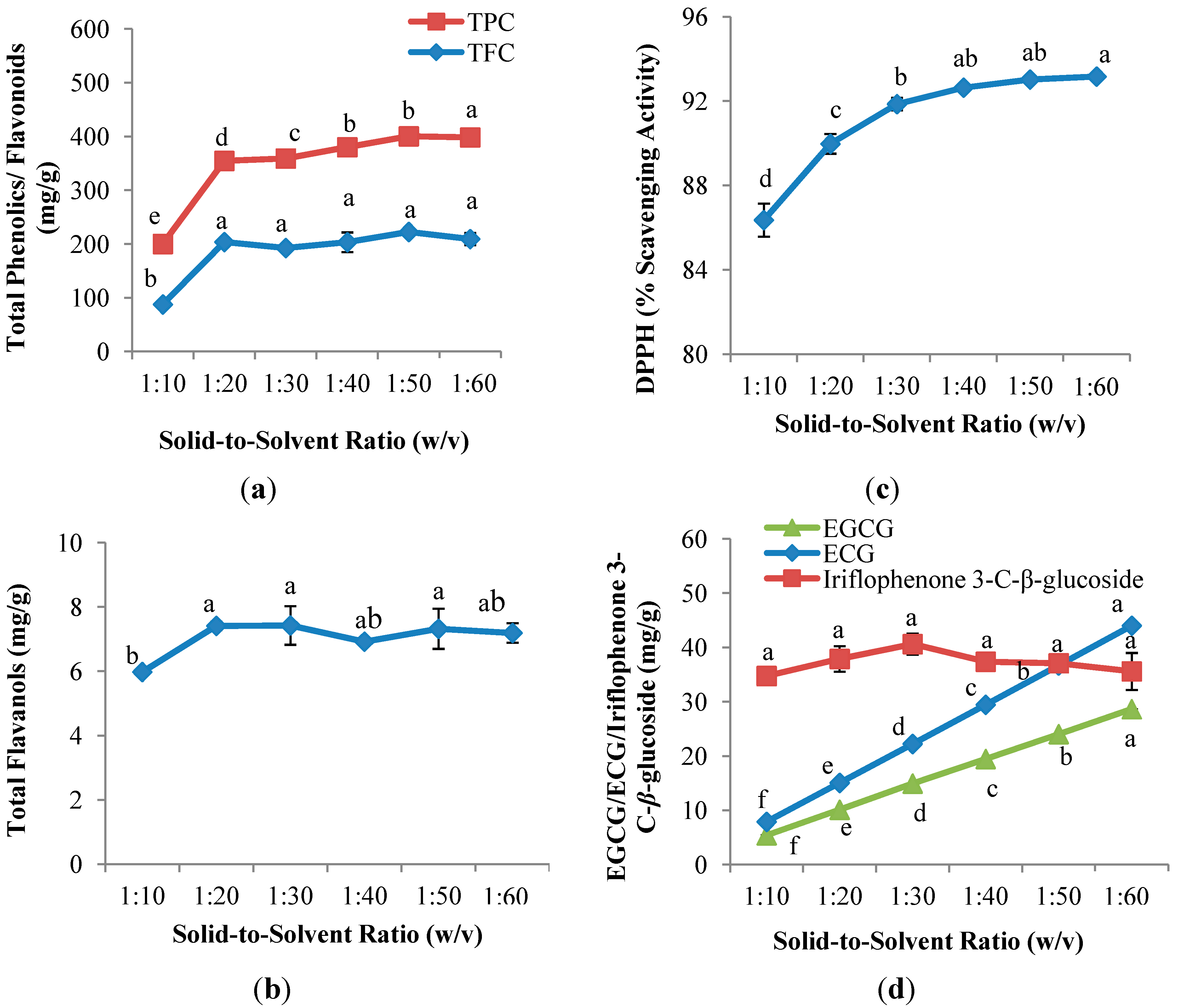
2.2.2. Antioxidant Capacity of the CPE of Aquilaria crassna
2.2.3. Quantitative and Qualitative Investigation of the CPE of Aquilaria crassna
2.3. Extraction Time
2.3.1. Polyphenol Content of the CPE of Aquilaria crassna
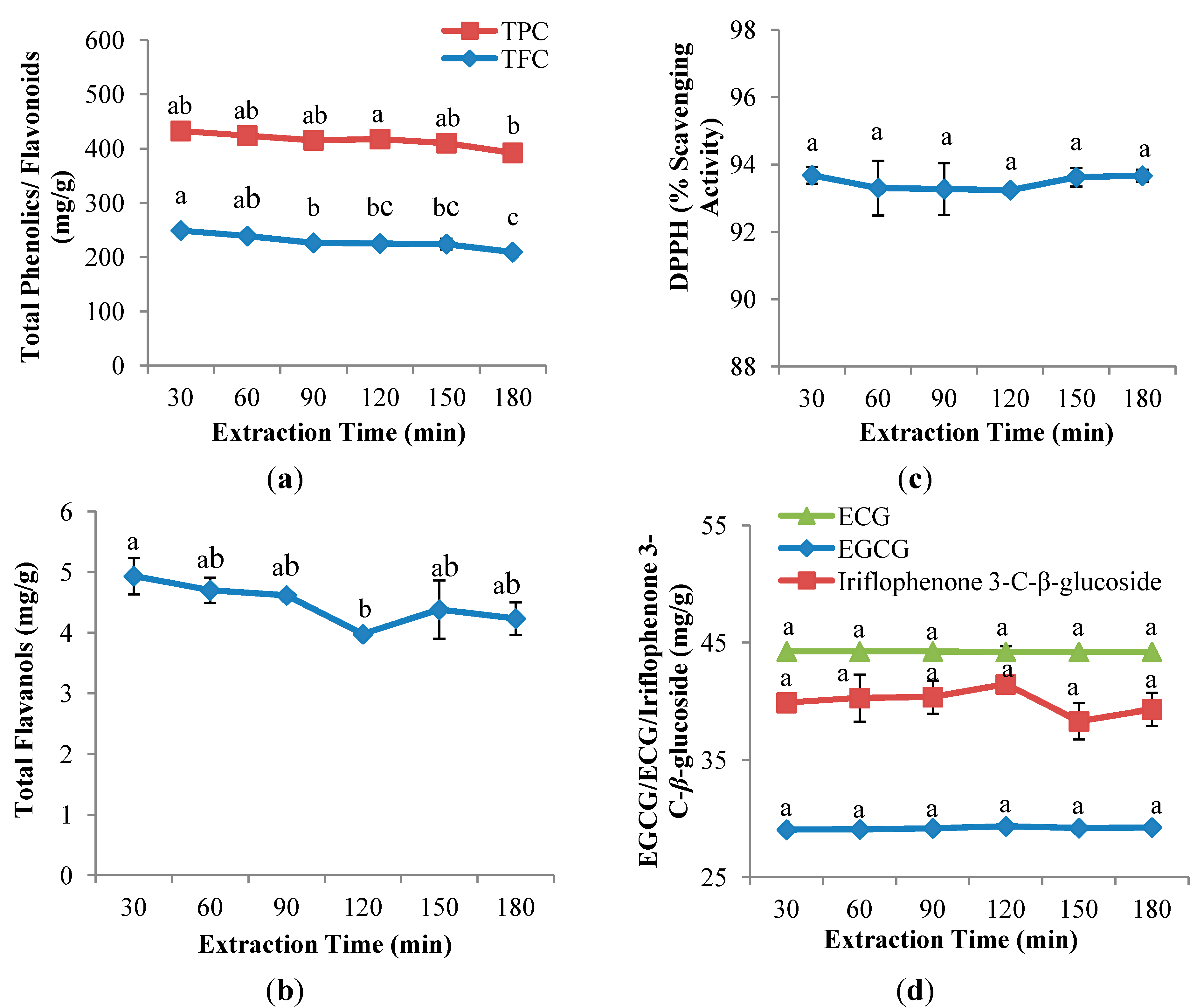
2.3.2. Antioxidant Capacity of the CPE of Aquilaria crassna
2.3.3. Quantitative and Qualitative Investigation of the CPE of Aquilaria crassna
2.4. Comparative Study with Reference Antioxidants
3. Experimental Section
3.1. Plant Material
3.2. Chemical Reagents
3.3. Sample Preparation
3.4. Solvent Extraction
3.5. Experimental Design
3.5.1. Ethanol Concentration
3.5.2. Solid-to-Solvent Ratio
3.5.3. Extraction Time
3.6. Comparative Study with Reference Antioxidants
3.6.1. Preparation of the IC50 (50% Inhibitory Concentration) Curve of the CPE from A. crassna
3.6.2. Preparation of IC50 Curves for Reference Antioxidants
3.7. Evaluation of Antioxidant Compounds
3.7.1. Determination of Total Phenolic Content (TPC)
3.7.2. Determination of Total Flavonoid Content (TFC)
3.7.3. Total Flavanol (TF) Assay
3.8. Evaluation of Antioxidant Capacity
DPPH Radical Scavenging Capacity
3.9. HPLC-DAD Analysis
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. London Ser. B 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Oreopoulou, V.; Tzia, C. Utilisation of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In Utilisation of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer Science + Business Media: New York, NY, USA, 2007; pp. 209–232. [Google Scholar]
- Hara, H.; Ise, Y.; Morimoto, N.; Shimazawa, M.; Ichihashi, K.; Ohyama, M.; Iinuma, M. Laxative effect of agarwood leaves and its mechanism. Biosci. Biotechnol. Biochem. 2008, 72, 335–345. [Google Scholar]
- Weiwitayaklung, P.; Thavanapong, N.; Charoenteeraboon, J. Chemical constituents and antimicrobial activity of essential oil and extracts of Heartwood of Aquilaria crassna obtained from water distillation and supercritical fluid carbon dioxide extraction. Silpakorn Univ. Sci. Technol. J. 2009, 3, 25–33. [Google Scholar]
- Oldfield, S.; Lusty, C.; MacKinven, A. The Word List of Threatened Trees; World Conservation Press: Cambridge, UK, 1998; p. 650. [Google Scholar]
- Barden, A.; Noordainie, A.A.; Mullihen, T.; Song, M. Heart of the Matter: Agarwood Use and Trade and CITIES Implementation for Aquilaria Malaccensis; TRAFFIC International: Cambridge, UK, 2000; p. 52. [Google Scholar]
- Rice-Evans, C. Flavonoids and isoflavones: Absorption, metabolism, and bioactivity. Free Radic. Biol. Med. 2004, 36, 827–828. [Google Scholar]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Ibrahim, M.; Jaafar, H. Abscisic acid induced changes in production of primary and secondary metabolites, photosynthetic capacity, antioxidant capability, antioxidant enzymes and lipoxygenase inhibitory activity of Orthosiphon stamineus benth. Molecules 2013, 18, 7957–7976. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Lee, L.-S.; Lee, N.; Kim, Y.; Lee, C.-H.; Hong, S.; Jeon, Y.-W.; Kim, Y.-E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Separation and quantification of flavonoids. In Flavonoids: Chemistry, Biochemistry, and Applications; Anderson, O.M., Markham, K.R., Eds.; CRC Press: New York, NY, USA, 2006; pp. 1–36. [Google Scholar]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Pinelo, M.; del Fabbro, P.; Marzocco, L.; Nunez, M.J.; Vicoli, M.C. Optimization of continuous phenol extraction from Vitis vinifera byproducts. Food Chem. 2005, 92, 109–117. [Google Scholar] [CrossRef]
- Chew, K.K.; Ng, S.Y.; Thoo, Y.Y.; Khoo, M.Z.; wan Aida, W.M.; Ho, C.W. Effect of ethanol concentration, extraction time, and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int. Food Res. J. 2011, 18, 566–573. [Google Scholar]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Debez, I.B.S.; Karoui, I.J.; Sellami, I.H.; Msaada, K.; Limam, F.; Marzouk, B. Effects of extraction solvents and provenances on phenolic contents and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Food Bioprocess Technol. 2012, 5, 2827–2836. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Gil, C.; Duarte, A.P. Antioxidant activity of extracts of Portuguese shrubs: Pterospartum tridentatum, Cytisus scoparius and Erica spp. J. Med. Plants Res. 2009, 3, 886–893. [Google Scholar]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar]
- Hismath, I.; wan Aida, W.M.; Ho, C.W. Optimization of extraction conditions for phenolic compounds from neem (Azadirachta indica) leaves. Int. Food Res. J. 2011, 18, 931–939. [Google Scholar]
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Tan, P.W.; Tan, C.P.; Ho, C.W. Antioxidant properties: Effect of solid-to-solvent ratio on antioxidant compounds and capacities of Pegaga (Centella asiatica). Int. Food Res. J. 2011, 18, 553–558. [Google Scholar]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Bi, H.-M.; Liu, C.-J. Extraction of bio-active components from Rhodiola sachalinensis under ultrahigh hydrostatic pressure. Sep. Purif. Technol. 2007, 57, 277–282. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar]
- Chan, S.W.; Lee, C.Y.; Yap, C.F.; wan Aida, W.M.; Ho, C.W. Optimization of extraction condition for phenolic compounds from limau purut (Citrus hystrix) peels. Int. Food Res. J. 2009, 16, 203–213. [Google Scholar]
- Herodež, Š.S.; Hadolin, M.; Škerget, M.; Knez, Ž. Solvent extraction study of antioxidants from Balm (Melissa officinalis L.) leaves. Food Chem. 2003, 80, 275–282. [Google Scholar] [CrossRef]
- Uma, D.B.; Ho, C.W.; wan Aida, W.M. Optimization of extraction parameters of total phenolic compounds from henna (Lawsonis intermis) leaves. Sains Malays. 2010, 39, 119–128. [Google Scholar]
- Kyi, T.M.; Daud, W.R.W.; Mohammad, A.B.; Samsudin, M.W.; Kadhum, A.A.H.; Talib, M.Z.M. The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans. Int. J. Food Sci. Technol. 2005, 40, 323–331. [Google Scholar] [CrossRef]
- Jeong, W.S.; Kong, A.N.T. Biological properties of monomeric and polymeric catechins: Green tea catechins and procyanidins. Pharm. Biol. 2004, 42, 84–93. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.; Barrow, C. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Evaluation of parameters that affect the 4-dimethylaminocinnamaldehyde assay for flavanols and proanthocyanidins. J. Food Sci. 2010, 75, C619–C625. [Google Scholar] [CrossRef]
- Sample Availability: Samples of crude extract from agarwood (Aquilaria crassna) young leaves are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tay, P.Y.; Tan, C.P.; Abas, F.; Yim, H.S.; Ho, C.W. Assessment of Extraction Parameters on Antioxidant Capacity, Polyphenol Content, Epigallocatechin Gallate (EGCG), Epicatechin Gallate (ECG) and Iriflophenone 3-C-β-Glucoside of Agarwood (Aquilaria crassna) Young Leaves. Molecules 2014, 19, 12304-12319. https://doi.org/10.3390/molecules190812304
Tay PY, Tan CP, Abas F, Yim HS, Ho CW. Assessment of Extraction Parameters on Antioxidant Capacity, Polyphenol Content, Epigallocatechin Gallate (EGCG), Epicatechin Gallate (ECG) and Iriflophenone 3-C-β-Glucoside of Agarwood (Aquilaria crassna) Young Leaves. Molecules. 2014; 19(8):12304-12319. https://doi.org/10.3390/molecules190812304
Chicago/Turabian StyleTay, Pei Yin, Chin Ping Tan, Faridah Abas, Hip Seng Yim, and Chun Wai Ho. 2014. "Assessment of Extraction Parameters on Antioxidant Capacity, Polyphenol Content, Epigallocatechin Gallate (EGCG), Epicatechin Gallate (ECG) and Iriflophenone 3-C-β-Glucoside of Agarwood (Aquilaria crassna) Young Leaves" Molecules 19, no. 8: 12304-12319. https://doi.org/10.3390/molecules190812304