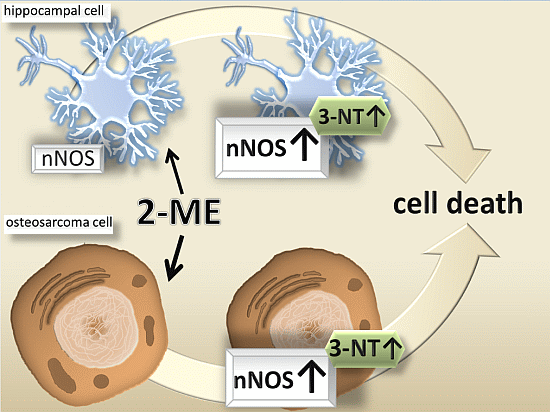
Neuronal Nitric Oxide Synthase Induction in the Antitumorigenic and Neurotoxic Effects of 2-Methoxyestradiol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anticancer Effects of 2-ME
2.1.1. Antiproliferative Properties of 2-ME

2.1.2. Effect of 2-ME and E2 on Induction of Cell Death in OS 143B

2.2. Neuronal-Type Signaling in OS Cells—nNOS as Molecular Target of 2-ME
2.2.1. Impact of 2-ME on nNOS and 3-NT Protein Levels

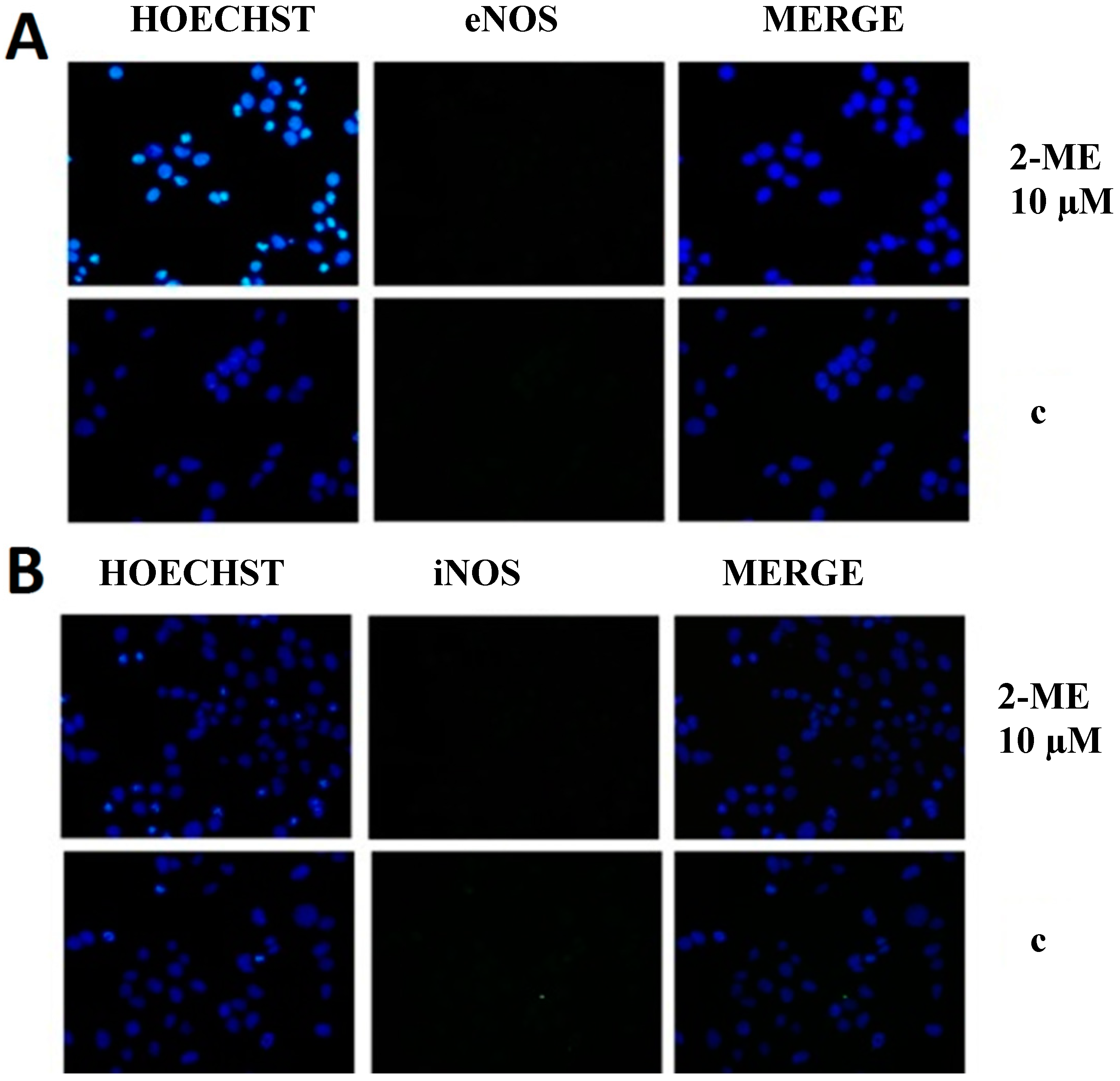

2.3. RNS Engaged in Anticancer Mechanism of 2-ME
Impact on Level of Nitric Oxide
3. Experimental Section
3.1. Reagents
3.2. Cell Line and Culture Conditions
3.3. Cell Viability Assay (MTT Assay)
3.4. Assessment of Apoptosis by Flow Cytometry with Double Annexin V—Propidium Iodide (PI) Staining
3.5. Assessment of Apoptosis by DNA Fragmentation ELISA Kit
3.6. Western Blotting
3.7. Immunofluorescence Microscopy
3.8. Statistical Analysis
4. Conclusions
Abbreviation
| c | Control |
| CNS | Central nervous system |
| DAF-2DA | Diaminofluorescein-2-diacetate |
| E2 | 17β-Estradiol |
| l-NDBA | Nω-Nitroarginine-2,4-l-diaminobutyric amide di(trifluoroacetate) salt |
| 2-ME | 2-Methoxyestradiol |
| nNOS | Neuronal nitric oxide synthase |
| 3-NT | 3-Nitrotyrosine |
| NMDA | N-Methyl-d-aspartate receptor |
| OS | Osteosarcoma |
| PD | Parkinson’s disease |
| SOD | Superoxide dismutase |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lethaby, C.D. A systematic review of time to diagnosis in children and young adults with cancer. Arch. Dis. Child. 2013, 98, 349–355. [Google Scholar] [CrossRef]
- Chi, S.N.; Conklin, L.S.; Qin, J.; Meyers, P.A.; Huvos, A.G.; Healey, J.H.; Gorlick, R. The patterns of relapse in osteosarcoma: The memorial sloan-kettering experience. Pediatr. Blood Cancer 2004, 42, 46–51. [Google Scholar] [CrossRef]
- Van Driel, M.; van Leeuwen, J.P. Cancer and bone: A complex. Arch. Biochem. Biophys. 2014, 18. [Google Scholar] [CrossRef]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar]
- Yamamoto, N.; Tsuchiya, H. Chemotherapy for osteosarcoma—Where does it come from? What is it? Where is it going? Expert Opin. Pharmacother. 2013, 14, 2183–2193. [Google Scholar]
- Yang, C.Y.; Chen, C.F.; Chen, W.M.; Wu, P.K.; Lee, F.T.; Chen, P.C.; Liu, C.L.; Chen, T.H. Osteoblastoma in the region of the hip. J. Chin. Med. Assoc. 2013, 76, 115–120. [Google Scholar]
- Yuan, W.; Yu, Y.; Li, J.; Singh, P.; Li, D.; Gui, Y.; Zheng, X.L. Estrogen metabolite 2-methoxyestradiol prevents hypertension in deoxycorticosterone acetate-salt rats. Cardiovasc. Drugs Ther. 2013, 27, 17–22. [Google Scholar]
- Peyrat, J.F.; Brion, J.-D.; Alami, M. Synthetic 2-methoxyestradiol derivatives: Structure-activity relationships. Curr. Med. Chem. 2012, 19, 4142–4156. [Google Scholar]
- Bruce, J.Y.; Eickhoff, J.; Pili, R.; Logan, T.; Carducci, M.; Arnott, J.; Treston, A.; Wilding, G.; Liu, G. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Investig. New Drugs 2012, 30, 794–802. [Google Scholar]
- Kulke, M.H.; Chan, J.A.; Meyerhardt, J.A.; Zhu, A.X.; Abrams, T.A.; Blaszkowsky, L.S.; Regan, E.; Sidor, C.; Fuchs, C.S. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother. Pharmacol. 2011, 68, 293–300. [Google Scholar]
- Harrison, M.R.; Hahn, N.M.; Pili, R.; Oh, W.K.; Hammers, H.; Sweeney, C.; Kim, K.; Perlman, S.; Arnott, J.; Sidor, C.; et al. A phase II study of 2-methoxyestradiol (2ME2), NanoCrystalA (R) dispersion (NCD) in patients with taxane-refractory metastatic castrate-resistant prostate cancer (CRPC). Investig. New Drugs 2011, 29, 1465–1474. [Google Scholar]
- Duncan, G.S.; Brenner, D.; Tusche, M.W.; Brüstle, A.; Knobbe, C.B.; Elia, A.J.; Mock, T.; Bray, M.R.; Krammer, P.H.; Mak, T.W. 2-Methoxyestradiol inhibits experimental autoimmune encephalomyelitis through suppression of immune cell activation. Proc. Natl. Acad. Sci. USA 2012, 109, 21034–21039. [Google Scholar]
- Benedikt, M.B.; Mahlum, E.W.; Shogren, K.L.; Subramaniam, M.; Spelsberg, T.C. 2-Methoxyestradiol regulates osteoprotegerin expression in osteosarcoma cells. J. Cell. Biochem. 2010, 109, 950–956. [Google Scholar]
- Maran, A.; Shogren, K.L.; Benedikt, M.; Sarkar, G.; Turner, R.T.; Yaszemski, M.J. 2-Methoxyestradiol-induced cell death in osteosarcoma cells is preceded by cell cycle arrest. J. Cell. Biochem. 2008, 104, 1937–1945. [Google Scholar]
- Maran, A.; Zhang, M.; Kennedy, A.M.; Sibonga, J.D.; Rickard, D.J.; Spelsberg, T.C.; Turner, R.T. 2-Methoxyestradiol induces interferon gene expression and apoptosis in osteosarcoma cells. Bone 2002, 30, 393–398. [Google Scholar] [CrossRef]
- Cicek, M.; Iwaniec, U.T.; Goblirsch, M.J.; Vrabel, A.; Ruan, M.; Clohisy, D.R.; Turner, R.R.; Oursler, M.J. 2-methoxyestradiol suppresses osteolytic breast cancer tumor progression in vivo. Cancer Res. 2007, 67, 10106–10111. [Google Scholar]
- Rajkumar, S.V.; Richardson, P.G.; Lacy, M.Q.; Dispenzieri, A.; Greipp, P.R.; Witzig, T.E.; Schlossman, R.; Sidor, C.F.; Anderson, K.C.; Gertz, M.A. Novel therapy with 2-methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin. Cancer Res. 2007, 13, 6162–6167. [Google Scholar] [CrossRef]
- Dahut, W.L.; Lakhani, N.J.; Gulley, J.L.; Arlen, P.M.; Kohn, E.C.; Kotz, H.; McNally, D.; Parr, A.; Nguyen, D.; Yang, S.X.; et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol. Ther. 2006, 5, 22–27. [Google Scholar] [CrossRef]
- Sweeney, C.; Liu, G.; Yiannoutsos, C.; Kolesar, J.; Horvath, D.; Staab, M.J.; Fife, K.; Armstrong, V.; Treston, A.; Sidor, C.; et al. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin. Cancer Res. 2005, 11, 6625–6633. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Anderson, R.L.; Hughes, R.A.; Altmann, E.; Schuliga, M.; Ziogas, J.; Stewart, A.G. 2-Methoxyestradiol—A unique blend of activities generating a new class of anti-tumour/anti-inflammatory agents. Drug Discov. Today 2007, 12, 577–584. [Google Scholar]
- Mueck, A.O.; Seeger, H. 2-Methoxyestradiol-Biology and mechanism of action. Steroids 2010, 75, 625–631. [Google Scholar]
- Seegers, J.C.; Lottering, M.L.; Grobler, C.J.; van Papendorp, D.H.; Habbersett, R.C.; Shou, Y.; Lehnert, B.E. The mammalian metabolite, 2-methoxyestradiol, affects p53 levels and apoptosis induction in transformed cells but not in normal cells. J. Steroid Biochem. Mol. Biol. 1997, 62, 253–267. [Google Scholar]
- West, A.B.; Dawson, V.L.; Dawson, T.M. To die or grow: Parkinson’s disease and cancer. Trends Neurosci. 2005, 28, 348–352. [Google Scholar]
- Picchio, M.C.; Martin, E.S.; Cesari, R.; Calin, G.A.; Yendamuri, S.; Kuroki, T.; Pentimalli, F.; Sarti, M.; Yoder, K.; Kaiser, L.R.; et al. Alterations of the tumor suppressor gene Parkin in non-small cell lung cancer. Clin. Cancer Res. 2004, 10, 2720–2724. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Murray, I.V.J.; Chen, Q.P.; Souza, J.M.; Hurtig, H.I.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M.Y. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 2000, 29, 985–989. [Google Scholar]
- Fujita, M.; Sugama, S.; Nakai, M.; Takenouchi, T.; Wei, J.S.; Urano, T.; Inoue, S.; Hashimoto, M. Alpha-synuclein stimulates differentiation of osteosarcoma cells—Relevance to down-regulation of proteasome activity. J. Biol. Chem. 2007, 282, 5736–5748. [Google Scholar]
- Foerstermann, U.; Sessa, C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar]
- Ischiropoulos, H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998, 356, 1–11. [Google Scholar]
- Ischiropoulos, H.; Beckman, J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Investig. 2003, 111, 163–169. [Google Scholar] [CrossRef]
- Blanchard-Fillion, B.; Prou, D.; Polydoro, M.; Spielberg, D.; Tsika, E.; Wang, Z.N.; Hazen, S.L.; Koval, M.; Przedborski, S.; Ischiropoulos, H. Metabolism of 3-nitrotyrosine induces apoptotic death in dopaminergic cells. J. Neurosci. 2006, 26, 6124–6130. [Google Scholar]
- Mihm, M.J.; Schanbacher, B.L.; Wallace, B.L.; Wallace, J.L.; Uretsky, N.J.; Bauer, J.A. Free 3-nitrotyrosine causes striatal neurodegeneration in vivo. J. Neurosci. 2001, 21, RC149. [Google Scholar]
- Kalariti, N.; Lembessis, P.; Koutsilieris, M. Characterization of the glutametergic system in MG-63 osteoblast-like osteosarcoma cells. Anticancer Res. 2004, 24, 3923–3929. [Google Scholar]
- Skerry, T.M.; Genever, P.G. Glutamate signalling in non-neuronal tissues. Trends Pharmacol. Sci. 2001, 22, 174–181. [Google Scholar]
- Tofighi, R.; Johansson, C.; Goldoni, M.; Ibrahim, W.N.; Gogvadze, V.; Mutti, A.; Ceccatelli, S. Hippocampal neurons exposed to the environmental contaminants methylmercury and polychlorinated biphenyls undergo cell death via parallel activation of calpains and lysosomal proteases. Neurotox. Res. 2011, 19, 183–194. [Google Scholar] [CrossRef]
- Liua, J.; Lib, L.; Suob, W.Z. HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci. 2009, 27, 267–271. [Google Scholar]
- Shi, C.; Zhu, X.M.; Wang, J.; Long, D. Intromitochondrial IκB/NF-κB signaling pathway is involved in amyloid β peptide-induced mitochondrial dysfunction. J. Bioenerg. Biomembr. 2014. [Google Scholar] [CrossRef]
- Siedlecka-Kroplewska, K.; Szczerba, A.; Lipinska, A.; Slebioda, T.; Kmiec, Z. 3-Fluoromethcathinone, a structural analog of mephedrone, inhibits growth and induces cell cycle arrest in HT22 mouse hippocampal cells. J. Physiol. Pharmacol. 2014, 65, 241–246. [Google Scholar]
- Picazo, O.; Azcoitia, I.; Garcia-Segura, L.M. Neuroprotective and neurotoxic effects of estrogens. Brain Res. 2003, 990, 20–27. [Google Scholar]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar]
- Tsukamoto, A.; Kaneko, Y.; Yoshida, T.; Han, K.; Ichinose, M.; Kimura, S. 2-Methoxyestradiol, an endogenous metabolite of estrogen, enhances apoptosis and beta-galactosidase expression in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1998, 248, 9–12. [Google Scholar]
- Yeo, W.S.; Kim, Y.J.; Kabir, M.H.; Kang, J.W.; Kim, K.P. Mass spectrometric analysis of protein tyrosine nitration in aging and neurodegenerative diseases. Mass Spectrom. Rev. 2014, 2. [Google Scholar] [CrossRef]
- Feeney, M.B.; Schöneich, C. Tyrosine modifications in aging. Antioxid. Redox Signal. 2012, 17, 1571–1579. [Google Scholar] [CrossRef]
- Bombeiro, A.L.; D’Império Lima, M.R.; Chadi, G.; Alvarez, J.M. Neurodegeneration and increased production of nitrotyrosine, nitric oxide synthase, IFN-gamma and S100beta protein in the spinal cord of IL-12p40-deficient mice infected with Trypanosoma cruzi. Neuroimmunomodulation 2010, 17, 67–78. [Google Scholar]
- Bitel, C.L.; Feng, Y.; Souayah, N.; Frederikse, P.H. Increased expression and local accumulation of the prion protein, Alzheimer Aβ peptides, superoxide dismutase 1, and nitric oxide synthases 1 & 2 in muscle in a rabbit model of diabetes. BMC Physiol. 2010, 10, 18. [Google Scholar]
- Pacher, P.; Mackie, K. Interplay of cannabinoid 2 (CB2) receptors with nitric oxide synthases, oxidative and nitrative stress, and cell death during remote neurodegeneration. J. Mol. Med. 2012, 90, 347–351. [Google Scholar] [CrossRef]
- Bobba, A.; Atlante, A.; Moro, L.; Calissano, P.; Marra, E. Nitric oxide has dual opposite roles during early and late phases of apoptosis in cerebellar granule neurons. Apoptosis 2007, 12, 1597–1610. [Google Scholar]
- Heigold, S.; Sers, C.; Bechtel, W.; Ivanovas, B.; Schafer, R.; Bauer, G. Nitric oxide mediates apoptosis induction selectively in transformed fibroblasts compared to nontransformed fibroblasts. Carcinogenesis 2002, 23, 929–941. [Google Scholar]
- Sullivan, R.; Graham, C.H. Chemosensitization of cancer by nitric oxide. Curr. Pharm. Des. 2008, 14, 1113–1123. [Google Scholar]
- Bayir, H.; Kagan, C.E.; Clark, R.S.B.; Janesko-Feldman, K.; Rafikov, R.; Huang, Z.T.; Zhang, X.J.; Vagni, V.; Billiar, T.R.; Kochanek, P.M. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J. Neurochem. 2007, 101, 168–181. [Google Scholar]
- Gorska, M.; Gammazza, A.M.; Zmijewski, M.A.; Campanella, C.; Cappello, F.; Wasiewicz, T.; Kuban-Jankowska, A.; Daca, A.; Sielicka, A.; Popowska, U.; et al. Geldanamycin-induced osteosarcoma cell death is associated with hyperacetylation and loss of mitochondrial pool of heat shock protein 60 (Hsp60). PLoS One 2013, 8, e71135. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (2-ME) are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gorska, M.; Kuban-Jankowska, A.; Zmijewski, M.A.; Gorzynik, M.; Szkatula, M.; Wozniak, M. Neuronal Nitric Oxide Synthase Induction in the Antitumorigenic and Neurotoxic Effects of 2-Methoxyestradiol. Molecules 2014, 19, 13267-13281. https://doi.org/10.3390/molecules190913267
Gorska M, Kuban-Jankowska A, Zmijewski MA, Gorzynik M, Szkatula M, Wozniak M. Neuronal Nitric Oxide Synthase Induction in the Antitumorigenic and Neurotoxic Effects of 2-Methoxyestradiol. Molecules. 2014; 19(9):13267-13281. https://doi.org/10.3390/molecules190913267
Chicago/Turabian StyleGorska, Magdalena, Alicja Kuban-Jankowska, Michal Aleksander Zmijewski, Monika Gorzynik, Michal Szkatula, and Michal Wozniak. 2014. "Neuronal Nitric Oxide Synthase Induction in the Antitumorigenic and Neurotoxic Effects of 2-Methoxyestradiol" Molecules 19, no. 9: 13267-13281. https://doi.org/10.3390/molecules190913267