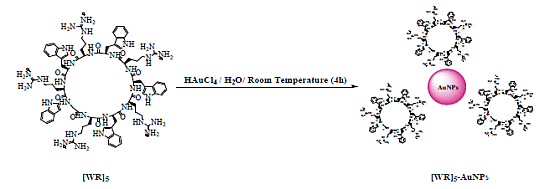Cyclic Peptide-Capped Gold Nanoparticles for Enhanced siRNA Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Cyclic Peptide
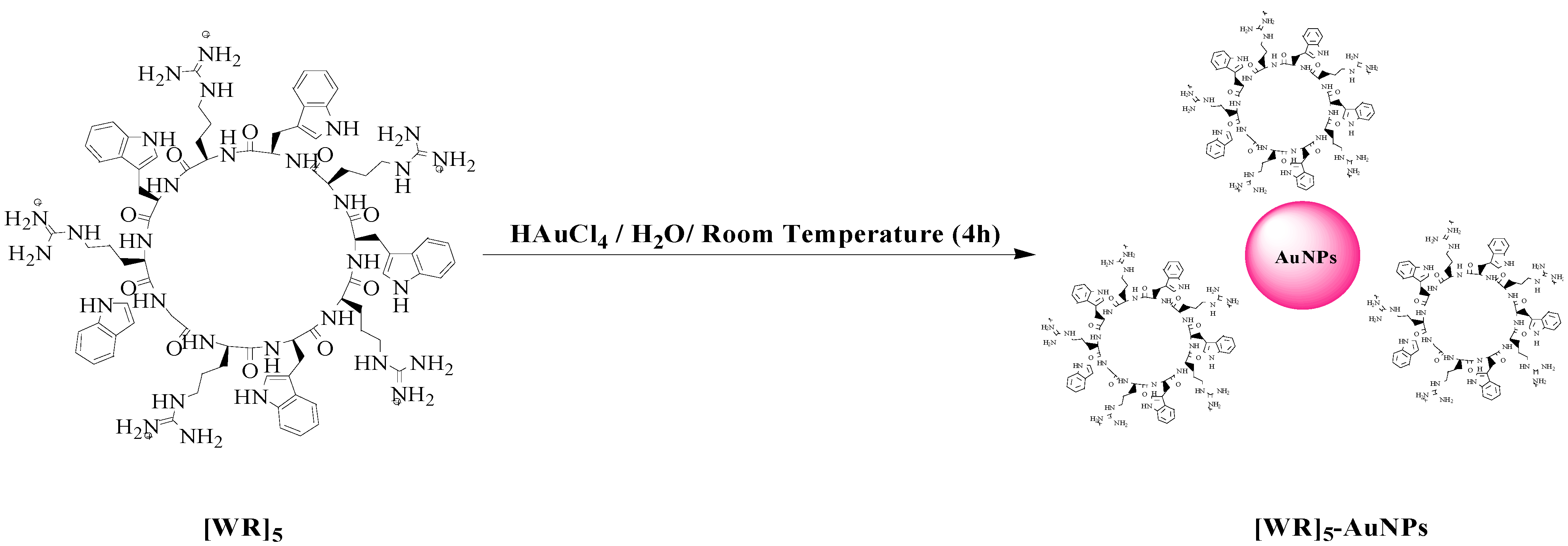

2.2. Synthesis of Peptide-Capped Gold Nanoparticles
2.3. Cytotoxicity of siRNA Delivery Systems

2.4. Cellular Uptake of F′-siRNA by Flow Cytometry

2.5. Cellular Uptake of F′-siRNA by Microscopy
2.6. Cellular Uptake of F'-[WR]5-AuNPs

3. Experimental Section
3.1. General Methods
3.2. Synthesis of [WR]5
3.3. Spectral Data
3.4. Synthesis of Peptide-Capped Gold Nanoparticles
3.5. Scanning Electron Microscopy (SEM)
3.6. Cell Culture
3.6.1. Cytotoxicity Assay
3.6.2. Microscopy Imaging
3.6.3. Flow Cytometry
3.6.4. Mechanism of Cellular Uptake when Endocytic Inhibitors Are Used
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sontheimer, E.J. Assembly and function of RNA silencing complexes. Nat. Rev. Mol. Cell Biol. 2005, 6, 127–138. [Google Scholar] [CrossRef]
- Song, E.; Lee, S.K.; Wang, J.; Ince, N.; Ouyang, N.; Min, J.; Chen, J.; Shankar, P.; Lieberman, J. RNA Interference targeting fas protects mice from Fulminant hepatitis. Nat. Med. 2003, 9, 347–351. [Google Scholar] [CrossRef]
- Sioud, M.; Sorensen, D.R. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem. Biophys. Res. Commun. 2003, 312, 1220–1225. [Google Scholar] [CrossRef]
- Urban-Klein, B.; Werth, S.; Abuharbeid, S.; Czubayko, F.; Aigner, A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in Vivo. Gene Ther. 2005, 12, 461–466. [Google Scholar] [CrossRef]
- Jantscha, J.; Turzac, N.; Volkea, M.; Eckardta, K.U.; Henseld, M.; Steinkasserer, A.; Willama, C.; Prechtel, A.T. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J. Immunol. Methods 2008, 337, 71–77. [Google Scholar] [CrossRef]
- Brummelkamp, T.R.; Bernards, R.; Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2002, 2, 243–247. [Google Scholar] [CrossRef]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Lieberman, J. The Silent Revolution: RNA Interference as basic biology, research tool, and therapeutic. J. Annul. Rev. Med. 2005, 56, 401–423. [Google Scholar] [CrossRef]
- Ryther, R.C.C.; Flynt, A.S.; Phillips, J.A.; Patton, J.G. siRNA therapeutics: big potential from small RNAs. Gene Ther. 2005, 12, 5–11. [Google Scholar]
- Rozema, D.B.; Lewis, D.L.; Wakefield, D.H.; Wong, S.C.; Klein, J.J.; Roesch, P.L.; Bertin, S.L.; Reppen, T.W.; Chu, Q.; Blokhin, A.V.; et al. Dynamic polyconjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 12982–12987. [Google Scholar]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004, 432, 173–178. [Google Scholar] [CrossRef]
- Pushparaj, P.N.; Melendez, A.J. Short interfering RNA (siRNA) as a novel therapeutic. Clin. Exp. Pharmacol. Physiol. 2006, 33, 504–510. [Google Scholar] [CrossRef]
- McCarrol, J.; Baigude, H.; Yang, C.S.; Rana, T.M. Nanotubes functionalized with lipids and natural amino acid dendrimers: A new strategy to create nanomaterials for delivering systemic RNAi. Bioconjug. Chem. 2010, 21, 56–63. [Google Scholar] [CrossRef]
- Behlke, M.A. Progress towards in vivo use of siRNAs. Mol. Ther. 2006, 13, 644–670. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, J.; Maronski, M.; Kotzbauer, P.T.; Lee, V.M.; Dichter, M.A.; Diamond, S.L. Non-classical nuclear localization signal peptides for high efficiency lipofection of primary neurons and neuronal cell lines. Neuroscience 2002, 112, 1–5. [Google Scholar]
- McManus, M.T.; Haines, B.B.; Dillon, C.P.; Whitehurst, C.E.; van Parijs, L.; Chen, J.; Sharp, P.A. Small interfering rna-mediated gene silencing in T lymphocytes. J. Immunol. 2002, 169, 5754–5760. [Google Scholar] [CrossRef]
- Strait, K.A.; Stricklett, P.K.; Kohan, J.L.; Miller, M.B.; Kohan, D.E. Identification of two Nuclear factor of activated T-cells (NFAT)-response elements in the 5'-upstream regulatory region of the ET-1 promoter. Am. J. Physiol. 2007, 293, 601–606. [Google Scholar]
- Wang, S.; Bui, V.; Hughes, J.A.; King, M.A.; Meyer, E.M. Adeno-associated virus mediated gene transfer into primary rat brain neuronal and glial cultures: Enhancement with the pH-sensitive surfactant dodecyl 2-(1wiimidazolyl) propionate. Neurochem. Int. 2000, 37, 1–6. [Google Scholar] [CrossRef]
- Ohki, E.C.; Tilkins, M.L.; Ciccarone, V.C.; Price, P.J. Improving the transfection efficiency of post-mitotic neurons. J. Neurosci. Methods 2001, 112, 95–99. [Google Scholar] [CrossRef]
- Mandal, D.; Nasrolahi Shirazi, A.; Parang, K. Cell-penetrating homochiral cyclic peptides as nuclear-targeting molecular transporters. Angew. Chem. Int. Ed. 2011, 50, 9633–9637. [Google Scholar] [CrossRef]
- Lewin, M.; Carlesso, N.; Tung, C.H.; Tang, X.W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef]
- Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjug. Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef]
- Liang, J.F.; Yang, V.C. Synthesis of doxorubicin-peptide conjugate with multidrug resistant tumor cell killing activity. Bioorg. Med. Chem. Lett. 2005, 15, 5071–5075. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Garlington, S.; Lin, Q.; Kirschberg, T.; Kreider, E.; McGrane, P.L.; Wender, P.A.; Khavari, P.A. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 2000, 6, 1253–1257. [Google Scholar] [CrossRef]
- Shirazi, N.A.; Tiwari, R.K.; Oh, D.; Banerjee, A.; Yadav, A.; Parang, K. Efficient delivery of cell impermeable phosphopeptides by a cyclic peptide amphiphile containing tryptophan and arginine. Mol. Pharm. 2013, 10, 2008–2020. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Rammohan, R.; Weissig, V.; Levchenko, T.S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 8786–8791. [Google Scholar] [CrossRef]
- Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 2006, 281, 3544–3551. [Google Scholar] [CrossRef]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Control. Release 2010, 148, 122–127. [Google Scholar] [CrossRef]
- Kumar, P.; Ban, H-S.; Kim, S-S; Wu, H.; Pearson, T.; Greiner, D-L.; Laouar, A.; Yao, J.; Haridas, V.; Habiro, K.; et al. T Cell-specific sirna delivery suppresses hiv-1 infection in humanized mice. Cell 2008, 134, 577–586. [Google Scholar] [CrossRef]
- Kumar, P.; Haoquan, W.; McBride, J.-L.; Jung, K.-E.; Kim, M.-H.; Davidson, B.-L.; Lee, S.-K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef]
- Oh, D.; Shirazi, N.A.; Northup, K.; Sullivan, B.; Tiwari, R.K.; Bisoffi, M.; Parang, K. Enhanced cellular uptake of short polyarginine peptides through fatty acylation and cyclization. Mol. Pharm. 2014, 11, 2845–2854. [Google Scholar] [CrossRef]
- Shirazi, N.A.; Tiwari, R.K.; Chhikara, B.S.; Mandal, D.; Parang, K. Design and biological evaluation of cell-penetrating peptide-doxorubicin conjugates as prodrugs. Mol. Pharm. 2013, 10, 488–499. [Google Scholar] [CrossRef]
- Shirazi, N.A.; Tiwari, R.K.; Oh, D.; Sullivan, B.; McCaffrey, K.; Mandal, D.; Parang, K. Surface decorated gold nanoparticles by linear and cyclic peptides as molecular transporters. Mol. Pharm. 2013, 10, 3137–3151. [Google Scholar] [CrossRef]
- Shirazi, N.A.; Mandal, D.; Tiwari, R.K.; Guo, L.; Lu, W.; Parang, K. Cyclic peptide-capped gold nanoparticles as drug delivery systems. Mol. Pharm. 2013, 10, 500–511. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors for a short period of time.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shirazi, A.N.; Paquin, K.L.; Howlett, N.G.; Mandal, D.; Parang, K. Cyclic Peptide-Capped Gold Nanoparticles for Enhanced siRNA Delivery. Molecules 2014, 19, 13319-13331. https://doi.org/10.3390/molecules190913319
Shirazi AN, Paquin KL, Howlett NG, Mandal D, Parang K. Cyclic Peptide-Capped Gold Nanoparticles for Enhanced siRNA Delivery. Molecules. 2014; 19(9):13319-13331. https://doi.org/10.3390/molecules190913319
Chicago/Turabian StyleShirazi, Amir Nasrolahi, Karissa L. Paquin, Niall G. Howlett, Dindyal Mandal, and Keykavous Parang. 2014. "Cyclic Peptide-Capped Gold Nanoparticles for Enhanced siRNA Delivery" Molecules 19, no. 9: 13319-13331. https://doi.org/10.3390/molecules190913319