Design, Synthesis and Fungicidal Activities of Some Novel Pyrazole Derivatives
Abstract
:1. Introduction
2. Result and Discussion
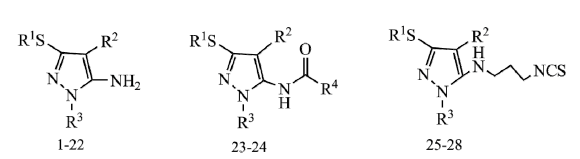
2.1. Chemical Synthesis

2.2. Fungicidal Activity
| Comp. | Inhibitory Rates (%) | |||||
|---|---|---|---|---|---|---|
| B. cinerea | R. solani | V. mali | T. cucumeris | F. oxysporum | F. graminearum | |
| 1 | 77.86 e | 79.30 d | 72.09 d | 67.87 d | 70.63 e | 51.25 g |
| 2 | 84.96 d | 98.02 b | 96.51 b | 88.78 c | 78.21 d | 91.00 c |
| 3 | 96.50 b | 98.44 b | 98.28 b | 98.87 b | 93.05 b | 98.05 b |
| 4 | 80.50 e | 93.38 c | 86.81 c | 85.73 c | 76.28 d | 73.00 d |
| 5 | 57.00 h | 66.98 f | 58.14 f | 50.12 g | 53.21 h | 67.07 e |
| 6 | 92.74 c | 97.25 b | 97.22 b | 96.13 b | 90.38 b | 98.05 b |
| 7 | 78.24 e | 79.19 d | 88.89 c | 60.62 ef | 71.79 e | 71.25 d |
| 8 | 85.00 d | 90.14 c | 94.19 b | 86.72 c | 71.79 e | 74.39 d |
| 9 | 47.36 j | 71.97 e | 84.30 c | 51.51 g | 35.90 k | 30.49 k |
| 10 | 92.14 c | 96.45 b | 95.83 b | 94.77 b | 84.56 c | 93.17 c |
| 11 | 62.00 g | 37.91 k | 72.22 d | 58.11 f | 36.54 k | 26.83 m |
| 12 | 49.50 j | 66.98 f | 48.26 g | 47.04 h | 32.05 l | 35.37 j |
| 13 | 52.44 i | 64.10 g | 65.23 e | 39.27 i | 65.79 g | 47.04 h |
| 14 | 40.22 k | 60.93 h | 21.62 j | 13.37 k | 48.03 i | 33.78 j |
| 15 | 65.49 g | 58.47 h | 66.22 e | 64.68 e | 68.42 f | 41.89 i |
| 16 | 41.40 k | 59.18 h | 34.12 i | 22.34 j | 36.18 k | 33.33 j |
| 17 | 33.86 m | 37.87 k | 37.87 i | 21.80 j | 35.53 k | 46.86 h |
| 18 | 34.42 m | 67.38 f | 52.05 g | 58.31 f | 42.11 j | 61.73 f |
| 19 | 36.19 l | 46.86 i | 41.89 h | 49.63 gh | 42.42 j | 46.97 h |
| 20 | 41.87 k | 62.21 h | 64.86 e | 57.73 f | 49.50 i | 52.33 g |
| 21 | 49.56 j | 65.23 fg | 51.35 g | 63.09 e | 42.17 j | 41.06 i |
| 22 | 36.74 l | 42.79 j | 33.78 i | 47.08 h | 36.01 k | 34.42 j |
| 23 | 41.32 k | 62.91 h | 43.24 h | 38.05 i | 44.74 j | 33.17 j |
| 24 | 65.49 g | 58.47 h | 66.22 e | 64.88 e | 68.42 f | 41.22 i |
| 25 | 42.42 k | 65.00 g | 39.19 h | 38.66 i | 67.07 f | 28.85 kl |
| 26 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a |
| 27 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a |
| 28 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a |
| 29 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 94.12b | 100.00 a |
| 30 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a |
| 31 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 100.00 a |
| 32 | 100.00 a | 100.00 a | 100.00 a | 100.00 a | 94.87 b | 92.48 c |
| Comp. | EC50 (95% FL) (μg/mL) | |||||
|---|---|---|---|---|---|---|
| B. cinerea | R. solani | V. mali | T. cucumeris | F. oxysporum | F. graminearum | |
| 1 | 10.627 (10.390–11.012) | 11.024 (10.736–11.369) | 12.822 (12.558–13.023) | -- | 15.320 (14.967–15.672) | -- |
| 2 | 8.073 (7.866–8.301) | 6.987 (6.735–7.207) | 4.622 (4.236–4.892) | 9.515 (9.256–9.791) | 13.588 (13.337–13.903) | 15.435 (15.049–15.793) |
| 3 | 5.848 (5.596–6.012) | 6.043 (5.757–6.401) | 3.738 (3.454–4.001) | 5.707 (5.351–6.065) | 9.515 (9.211–9.808) | 14.793 (14.522–15.001) |
| 4 | 9.891 (9.605–10.245) | 8.991 (8.076–9.799) | 5.707 (5.454–5.992) | 10.253 (9.897–10.711) | 13.859 (13.512–14.115) | 19.162 (18.908–19.354) |
| 6 | 7.233 (7.008–7.519) | 6.519 (6.262–6.804) | 3.757 (3.471–4.115) | 6.096 (5.792–6.454) | 9.699 (9.331–9.976) | 15.002 (14.879–15.226) |
| 7 | 10.641 (10.255–11.003) | 11.877 (11.631–12.012) | 6.043 (5.801–6.337) | -- | 15.053 (14.776–15.399) | 19.701 (19.319–19.962) |
| 8 | 8.221 (7.994–8.477) | 8.037 (7.811–8.362) | 4.987 (4.662–5.113) | 9.891 (9.612–10.055) | 14.793 (14.421–15.004) | 19.114 (18.875–19.336) |
| 9 | -- | 13.327 (12.942–13.785) | 5.314 (5.065–5.629) | -- | -- | -- |
| 10 | 7.493 (7.206–7.711) | 6.748 (6.454–6.976) | 4.455 (4.201–4.669) | 6.519 (6.233–6.877) | 10.668 (10.332–10.987) | 15.320 (15.066–15.632) |
| 11 | -- | -- | 12.877 (12.491–13.335) | -- | --- | -- |
| 26 | 2.432 (2.087–2.713) | 2.182 (1.824–2.568) | 1.787 (1.489–2.102) | 1.638 (1.342–1.940) | 6.986 (6.604–7.468) | 6.043 (5.757–6.401) |
| 27 | 3.742 (3.356–4.104) | 3.501 (3.115–3.859) | 1.919 (1.765–2.082) | 2.383 (2.057–2.612) | 8.073 (7.753–8.412) | 10.266 (9.982–10.624) |
| 28 | 3.870 (3.484–4.228) | 3.619 (3.233–3.977) | 2.432 (2.234–2.701) | 2.570 (2.244–2.841) | 8.221 (7.855–8.579) | 10.688 (10.302–11.064) |
| 29 | 4.843 (4.578–5.013) | 5.897 (5.514–6.022) | 3.330 (2.994–3.688) | 3.181 (2.880–3.405) | 9.171 (8.862–9.409) | 12.080 (11.804–12.311) |
| 30 | 4.006 (3.720–4.364) | 4.148 (3.762–4.506) | 2.760 (2.512–2.993) | 2.989 (2.703–3.347) | 8.359 (8.809–8.852) | 11.088 (10.702–11.464) |
| 31 | 4.297 (3.911–4.655) | 4.455 (4.179–4.722) | 2.989 (2.717–3.200) | 3.083 (2.811–3.338) | 8.665 (8.379–9.023) | 11.329 (10.943–11.697) |
| 32 | 4.994 (4.703–5.255) | 6.097 (5.882–6.303) | 3.458 (3.072–3.816) | 3.390 (3.146–3.707) | 9.339 (9.067–9.713) | 15.053 (14.769–15.342) |
| Carbendazole | 1.565 (1.328–1.779) | 1.420 (1.266–1.637) | 0.859 (0.692–1.006) | 1.253 (1.007–1.469) | 2.813 (2.582–3.011) | 2.262 (2.007–2.522) |
3. Experimental Section
3.1. General Information
3.2. Chemical Synthesis
3.2.1. General Procedure for the Synthesis of Compounds 3a–d
3.2.2. General Procedure for the Preparation of Compounds 1–26
3.2.3. General Procedure for Compounds 27–28
3.2.4. General Procedure for Compounds 29–32
3.3. Bioassay of Antifungal Activity
3.3.1. Preparation of Tested Fungal Pathogens
3.3.2. Fungicidal Activity Assay
3.3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Köller, W.; Scheinpflug, H. Fungal resistance to sterol biosynthesis inhibitors: A new challenge. Plant Dis. 1987, 71, 1066–1074. [Google Scholar]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 14, 186–194. [Google Scholar]
- Savary, S.; Fick, A.; Aubertot, J.N.; Hollier, C. Crop losses due to disease and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar]
- Wang, H.; Ren, S.X.; He, Z.Y.; Yan, X.N.; Feng, J.T.; Zhang, X. Synthesis, antifungal activities and qualitative structure: Activity relationship of carabrone hydrazone derivatives as potential antifungal agents. Int. J. Mol. Sci. 2014, 15, 4257–4272. [Google Scholar]
- Ypema, H.L.; Gold, R.E. Modification of a naturally occurring compound to produce a new fungicide. Plant Dis. 1999, 83, 4–19. [Google Scholar]
- Oercke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar]
- Chen, H.; Li, Z.; Han, Y. Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl (alkylthio)-5-pyrazole-1,3,4-oxadia-zoles (thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315. [Google Scholar]
- Meaza, G.; Bettarini, F.; Porta, L.P.; Piccardi, P.; Signorini, E.; Portoso, D.; Fornara, L. Synthesis and herbicide activity of novel heterocyclic protoporphyrinogen oxidase inhibitors. Pest Manag. Sci. 2004, 60, 1178–1188. [Google Scholar]
- Park, H.; Lee, K.; Park, S.; Ahn, B.; Lee, J.; Cho, H.Y.; Lee, K. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef]
- Finkelstein, B.L.; Strock, C.J. Synthesis and insecticidal activity of novel pyrazole methanesulfonates. Pestic. Sci. 1997, 50, 324–328. [Google Scholar]
- Fu, C.R.; Pei, J.; Ning, Y.; Liu, M.; Shan, P.C.; Liu, J.; Li, Y.Q.; Hu, F.Z.; Zhu, Y.Q.; Yang, H.Z.; et al. Synthesis and insecticidal activities of novel pyrazole oxime ether derivatives with different substituted pyridyl rings. Pest Manag. Sci. 2013, 70, 1207–1214. [Google Scholar]
- Wu, J.; Song, B.A.; Hu, D.Y.; Yue, M.; Yang, S. Design, synthesis and insecticidal acticities of novel pyrazole amides containing hydrazine substructures. Pest. Manag. Sci. 2012, 68, 801–810. [Google Scholar]
- Song, H.J.; Liu, Y.X.; Xiong, L.X.; Li, Y.Q.; Yang, N.; Wang, Q.M. Design, synthesis, and insecticidal activity of novel pyrazole derivatives containing α-hydroxymethyl-N-benzyl carboxamide, α-chloromethyl-N-benzyl carboxamide, and 4,5-dihydrooxazole moieties. J. Agric. Food. Chem. 2012, 60, 1470–1479. [Google Scholar]
- Li, M.; Liu, C.L.; Yang, J.C.; Zhang, J.B.; Li, Z.N.; Zhang, H.; Li, Z.M. Synthesis and biological activity of new (E)-α-(methoxyimino)benzeneacetate derivatives containing a substituted pyrazole ring. J. Agric. Food Chem. 2010, 58, 2664–2667. [Google Scholar] [CrossRef]
- Vicebtini, C.B.; Romangnoli, C.; Andreotti, E.; Mares, D. Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi. J. Agric. Food Chem. 2007, 55, 10331–10338. [Google Scholar]
- Hashimoto, S.; Iihama, T.; Kasahara, I.; Sano, S.; Sugiura, T. Pyrazole Derivatives and Agrohorticultural Bactericide Containing Same. WO Patent 1993007138, 1993. [Google Scholar]
- Hagiwara, K.; Suzuki, H. Preparation of Pyrazole Derivatives as Argrochemical Fungicide Insecticides and Acaricides. JP Patent 08193067, 1996. [Google Scholar]
- Fujii, K.; Tallaka, T. Preparation of Pyrazole Derivatives as Agrochemical Fungicides. JP Patent 07285942, 1995. [Google Scholar]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing oxime ethers moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar]
- Ouyang, G.P.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing oxime esters group. Bioorg. Med. Chem. 2008, 16, 9699–9707. [Google Scholar]
- Clemens, L. Pyrazole chemistry in crop protection. Heterocycles 2007, 71, 1467–1502. [Google Scholar]
- Deng, X.H.; Mani, N.S. Reaction of N-monosubstituted hydrazones with nitroolefins: A novel regioselective pyrazole synthesis. Org. Lett. 2006, 8, 3505–3508. [Google Scholar]
- Wu, H.; Zhang, X.; Zhang, G.A.; Zeng, S.Y.; Lin, K.C. Antifungal vapour-phase activity of a combination of allyl isothiocyanate and ethyl isothiocyanate against Botrytis cinerea and Penicillium expansum infection on apples. J. Phytopathol. 2011, 159, 450–455. [Google Scholar]
- Li, M.; Liu, C.L.; Li, L.; Yang, H.; Li, Z.N.; Zhang, H.; Li, Z.M. Design, synthesis and biological activities of new strobilurin derivatives containing substituted pyrazole. Pest Manag. Sci. 2010, 66, 107–112. [Google Scholar]
- Elbe, H.L.; Astrid, M.M.; Klaus, S.; Kuck, K.H.; Martin, K.; Thomas, J. Pyrazole Caroxanilide Fungicide. U.S. Patent 6369093, 2002. [Google Scholar]
- Ammermann, E.; Harries, V.; Kristgen, R.; König, H.; Lorenz, G.; Sauter, H. Pyrazole Derivatives, Their Preparation and Their Use as Pesticide. EP Patent 0691332, 1996. [Google Scholar]
- Nguyen, D.L. Use of 1-Phenyl Pyrazole Compounds. FR Patent 2745467, 1997. [Google Scholar]
- Dunkel, R.; Elbe, H.-L.; Kuck, K.-H.; Rieck, H.; Wachendorff-Neumann, U. Disubstituted Pyrazolylcarboxanilides. WO Patent 2003070705, 2003. [Google Scholar]
- Usui, Y.; Tsutsumi, Y. Preparation of Sulfamoyl Triazole Derivatives as Agrochemical Fungicides. JP Patent 07215971, 1995. [Google Scholar]
- Duvert, P.; Ngugen, D.L. Use of 1-Phenyl Pyrazole Compounds. FR Patent 2745466, 1997. [Google Scholar]
- Suzuki, H.; Hanaue, M.; Nishikubo, M. Preparation of Pyrazole Carboxamides Derivatives as Agrochemical Fungicides. JP Patent 91236368, 1992. [Google Scholar]
- Okada, I.; Okui, S.; Nakajima, T. Preparation of N-Benzyl-5-carbamoylpyrazole Derivatives as Agrochemical and Horticulture Fungicides. JP Patent 91206709, 1991. [Google Scholar]
- Zhao, W.G.; Wang, S.H.; Wang, W.Y. Improved synthesis of disulfide acetal chemical reagents. Chem. Reag. 2000, 22, 376–377. [Google Scholar]
- Wu, H.; Feng, J.T.; Lin, K.C.; Zhang, X. Synthesis and herbicidal activity of substituted pyrazole isothiocyanates. Molecules 2012, 17, 12187–12196. [Google Scholar]
- Feng, J.T.; Wang, H.; Ren, S.X.; He, J.; Liu, Y.; Zhang, X. Synthesis and antifungal activities of carabrol ester derivatives. J. Agric. Food Chem. 2012, 60, 3817–3823. [Google Scholar]
- Sangwan, N.K.; Dhindsa, K.S.; Malik, O.P.; Malik, M.S. 1-Acyl-3-(mono/disubstituted phenyl)-4-(H or methyl)-5-aryl-4,5-dihydropyrazoles ad potential antimicrobial agents. Chim. Acta Turc. 1983, 11, 65–72. [Google Scholar]
- Chen, G.Y.; Zhou, Y.W.; Cai, C.L.; Lu, J.; Zhang, X. Synthesis and antifungal activity of benzamidine derivatives carrying 1,2,3-triazole moieties. Molecules 2014, 19, 5674–5691. [Google Scholar]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.-R.; Wu, H.; He, Z.-Y.; Ma, Z.-Q.; Feng, J.-T.; Zhang, X. Design, Synthesis and Fungicidal Activities of Some Novel Pyrazole Derivatives. Molecules 2014, 19, 14036-14051. https://doi.org/10.3390/molecules190914036
Liu X-R, Wu H, He Z-Y, Ma Z-Q, Feng J-T, Zhang X. Design, Synthesis and Fungicidal Activities of Some Novel Pyrazole Derivatives. Molecules. 2014; 19(9):14036-14051. https://doi.org/10.3390/molecules190914036
Chicago/Turabian StyleLiu, Xue-Ru, Hua Wu, Ze-Yu He, Zhi-Qing Ma, Jun-Tao Feng, and Xing Zhang. 2014. "Design, Synthesis and Fungicidal Activities of Some Novel Pyrazole Derivatives" Molecules 19, no. 9: 14036-14051. https://doi.org/10.3390/molecules190914036