Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects
Abstract
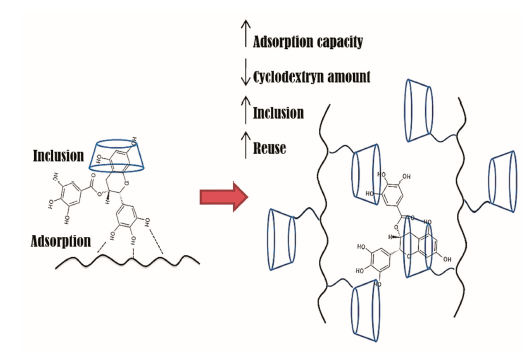
:1. Introduction

2. Polymers as Ternary Complex Constituents
3. Networks of Polymers and Cyclodextrins

4. Molecularly Imprinted Polymers (MIPs) with Cyclodextrins

5. Current Applications of Polymers and CD Networks
| Polymer | Application | Ref. | |
|---|---|---|---|
| β- and γ-CDs linked with chitosan | Bitter-masking of caffeine solutions and bitter natural extracts | [37] | |
| β-CD with one of the following crosslinking agents: epichlorohydrin, diphenyl carbonate, or hexamethylene diisocyanate and propargyl-β-CD with 1,3-bis(azidomethyl) benzene | Debittering agents by adsorption of narangin, limonin and caffeine | [38] | |
| β-CD and epichlorohydrin | Environmental purposes: detoxification of wastewater, color removal, concentration and purification of solutions | [19] | |
| Delivery of Lavandula angustifolia essential oil used as ambient odors | [39] | ||
| Retention of aroma compounds of Mentha piperita esential oil | [40] | ||
| Adsorption of pesticides from water | [41] | ||
| Removal of Cu2+ from aqueous solutionss | [42] | ||
| β-CD and anionic and cationic acrylamide | Enhancement of oil recovery | [22] | |
| Chemically cross-linked and grafted cyclodextrins | Drug release from hydrogels | [28] | |
| α-, β- and γ-CDs functionalized with acrylic groups | Aqueous nanogels | [43] | |
| β-CD polymer/tungsten carbide | Adsorption of rutin | [26] | |
| β-CD, acrylic acid, N,N'-methylenebisacrylamide | Hydrogel for the removal of heavy metal ions | [44] | |
| Monomethacrylated β-CD copolymerized with N-isopropylacrylamide | Drug delivery | [21] | |
| Oligosaccharide γ-CD with dibasic acid dichlorides | Adsorption of polychlorobiphenyl contaminants in oil | [45] | |
| β-CD-citric acid | Drug delivery of ciprofloxacin (an antibiotic) and prednisolone (an anti-inflammatory drug) | [46] | |
| β-CD-dextran polymers | Drug delivery | [47] | |
| HPγ-CD polymer and sulfobutylether-β-CD with epichlorohydrin | Adsorption of ionizable oxytetracycline antibiotics | [48] | |
| Phosphorous-containing β-CD polymers | 1-adamantyl acetic acid, or with divalent cations, such as Ca2+ | [49] | |
| CD crosslinked with 4,4'-methylenebis(phenyl isocyanate) | Removal of patulin from apple juice | [50] | |
| Glycidyl methacrylate alone or in combination with β-CD | Incorporation of insecticide in cotton textiles | [51] | |
| α-CD-polymer gels with various poly(ethylene oxide) (PEO)-based copolymers | Delivery of vancomycin for the treatment of bone infections | [52] | |
| From β-CD and sulfobutylether-β-CD with epichlorohydrin | Solubilization of repaglinide (hypoglycemic agent) | [53] | |
| β-CD-ionic liquid polymer with 1-benzylimidazole and crosslinked using toluenediisocyanate | Sorption capacity and high removal towards phenols and arsenic(V) | [54] | |
| Carboxymethyl-hydroxypropyl-β-CD polymer-modified Fe3O4 magnetic particles (CM-HP-β-CDCP-MNPs) | Magnetic solid phase extraction of rutin | [55] | |
| Crosslinked polyvinyl alcohol/glutaraldehyde (PVA/GA) membranes with β-CD | Textile liquid waste processing | [56] | |
| PCCA thin film modified with β-CD polymer | Capping cavity for the selective detection of paraoxon-ethyl and parathion-ethyl chemical agents | [57] | |
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar]
- Acuña-Rougier, C.; Olea-Azar, C. Thermodynamic and geometric study of diasteroisomeric complexes formed by racemic flavanones and three cyclodextrins through NMR. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 119–136. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocolloid 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.; Másson, M. Role of cyclodextrins in improving oral drug delivery. Am. J. Drug. Deliv. 2004, 2, 261–275. [Google Scholar] [CrossRef]
- Harada, A. Construction of supramolecular structures from cyclodextrins, polymers. Carbohyd. Polym. 1997, 34, 183–188. [Google Scholar] [CrossRef]
- Lu, J.; Mirau, P.A.; Tonelli, A.E. Chain conformations and dynamics of crystalline polymers as observed in their inclusion compounds by solid-state NMR. Prog. Polym. Sci. 2002, 27, 357–401. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Double-stranded inclusion complexes of cyclodextrin threaded on poly(ethylene glycol). Nature 1994, 370, 126–128. [Google Scholar] [CrossRef]
- Faucci, M.T.; Mura, P. Effect of water-soluble polymers on naproxen complexation with natural and chemically modified beta-cyclodextrins. Drug Dev. Ind. Pharm. 2001, 27, 909–917. [Google Scholar]
- Fenyvesi, É. Cyclodextrin polymers in the pharmaceutical industry. J. Incl. Phenom. 1988, 6, 537–545. [Google Scholar] [CrossRef]
- Szeman, J.; Fenyvesi, E.; Szejtli, J.; Ueda, H.; Machida, Y.; Nagai, T. Water soluble cyclodextrin polymers: Their interaction with drugs. J. Incl. Phenom. 1987, 5, 427–431. [Google Scholar] [CrossRef]
- Renard, E.; Deratani, A.; Volet, G.; Sebille, B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 1997, 33, 49–57. [Google Scholar] [CrossRef]
- Danel, C.; Azaroual, N.; Chavaria, C.; Odou, P.; Martel, B.; Vaccher, C. Comparative study of the complex forming ability and enantioselectivity of cyclodextrin polymers by CE and 1H-NMR. Carbohyd. Polym. 2013, 92, 2282–2292. [Google Scholar] [CrossRef]
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-soluble polymers and gels from the polycondensation between cyclodextrins and poly(carboxylic acid)s: A study of the preparation parameters. J. Appl. Polym. Sci. 2005, 97, 433–442. [Google Scholar]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Tonelli, A.E. Cyclodextrins as a means to nanostructure and functionalize polymers. J. Incl. Phenom. Macrocycl. Chem. 2007, 60, 197–202. [Google Scholar] [CrossRef]
- Munteanu, M.; Choi, S.; Ritter, H. Cyclodextrin Methacrylate via Microwave-Assisted Click Reaction. Macromolecules 2008, 41, 9619–9623. [Google Scholar] [CrossRef]
- Zhou, J.; Ritter, H. Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 2010, 1, 1552–1559. [Google Scholar] [CrossRef]
- Doyagüez, E.G.; Rodríguez-Hernández, J.; Corrales, G.; Fernández-Mayoralas, A.; Gallardo, A. Water-Soluble Pendant Copolymers Bearing Proline and Permethylated β-Cyclodextrin: pH-Dependent Catalytic Nanoreactors. Macromolecules 2012, 45, 7676–7683. [Google Scholar] [CrossRef]
- Moers, C.; Nuhn, L.; Wissel, M.; Stangenberg, R.; Mondeshki, M.; Berger-Nicoletti, E.; Thomas, A.; Schaeffel, D.; Koynov, K.; Klapper, M.; et al. Supramolecular linear-g-hyperbranched graft polymers: Topology and binding strength of hyperbranched side chains. Macromolecules 2013, 46, 9544–9553. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, D.-Q.; Wang, Y.-C.; Yao, S.-J. A novel β-cyclodextrin polymer/tungsten carbide composite matrix for expanded bed adsorption: Preparation and characterization of physical properties. Carbohyd. Polym. 2010, 80, 1085–1090. [Google Scholar]
- Zhao, J.; Lin, D.-Q.; Yao, S.-J. Adsorption of rutin with a novel β-cyclodextrin polymer adsorbent: Thermodynamic and kinetic study. Carbohyd. Polym. 2012, 90, 1764–1770. [Google Scholar] [CrossRef]
- Tonelli, A.E. Nanostructuring and functionalizing polymers with cyclodextrins. Polymer 2008, 49, 1725–1736. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted materials as advanced excipients for drug delivery systems. In Biotechnology Annual Review; El-Gewely, M.R., Ed.; Elsevier: Clayton, Australia, 2006; Volume 12, pp. 225–268. [Google Scholar]
- Asanuma, H.; Akiyama, T.; Kajiya, K.; Hishiya, T.; Komiyama, M. Molecular imprinting of cyclodextrin in water for the recognition of nanometer-scaled guests. Anal. Chim. Acta 2001, 435, 25–33. [Google Scholar] [CrossRef]
- Egawa, Y.; Shimura, Y.; Nowatari, Y.; Aiba, D.; Juni, K. Preparation of molecularly imprinted cyclodextrin microspheres. Int. J. Pharm. 2005, 293, 165–170. [Google Scholar] [CrossRef]
- Guo, M.; Hu, X.; Fan, Z.; Liu, J.; Wang, X.; Wang, Y.; Mi, H. Imprinted polymers with cyclodextrin pseudo-polyrotaxanes as pseudo-supports for protein recognition. Talanta 2013, 105, 409–416. [Google Scholar]
- Kyzas, G.Z.; Lazaridis, N.K.; Bikiaris, D.N. Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohyd. Polym. 2013, 91, 198–208. [Google Scholar] [CrossRef]
- Ng, S.M.; Narayanaswamy, R. Molecularly imprinted β-cyclodextrin polymer as potential optical receptor for the detection of organic compound. Sens. Actuator B-Chem. 2009, 139, 156–165. [Google Scholar] [CrossRef]
- Roche, P.J.R.; Ng, S.M.; Narayanaswamy, R.; Goddard, N.; Page, K.M. Multiple surface plasmon resonance quantification of dextromethorphan using a molecularly imprinted β-cyclodextrin polymer: A potential probe for drug–drug interactions. Sens. Actuator B-Chem. 2009, 139, 22–29. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Syu, M.-J. Synthesis of creatinine-imprinted poly(β-cyclodextrin) for the specific binding of creatinine. Biomaterials 2005, 26, 2759–2766. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Fang, H.-X.; Yu, L.-P. Molecularly imprinted photonic polymer based on β-cyclodextrin for amino acid sensing. Talanta 2013, 116, 283–289. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan-cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Frag. J. 2004, 19, 394–400. [Google Scholar] [CrossRef]
- Binello, A.; Robaldo, B.; Barge, A.; Cavalli, R.; Cravotto, G. Synthesis of cyclodextrin-based polymers and their use as debittering agents. J. App. Polym. Sci. 2008, 107, 2549–2557. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohyd. Polym. 2012, 87, 1963–1970. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297. [Google Scholar] [CrossRef]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef]
- Sikder, M.T.; Islam, M.S.; Kikuchi, T.; Suzuki, J.; Saito, T.; Kurasaki, M. Removal of Copper Ions From Water Using Epichlorohydrin Cross-Linked β-Cyclodextrin Polymer: Characterization, Isotherms and Kinetics. Water Environ. Res. 2014, 86, 296–304. [Google Scholar] [CrossRef]
- Kettel, M.J.; Dierkes, F.; Schaefer, K.; Moeller, M.; Pich, A. Aqueous nanogels modified with cyclodextrin. Polymer 2011, 52, 1917–1924. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. 2013, 53, 615–630. [Google Scholar]
- Kawano, S.; Kida, T.; Miyawaki, K.; Noguchi, Y.; Kato, E.; Nakano, T.; Akashi, M. Cyclodextrin polymers as highly effective adsorbents for removal and recovery of polychlorobiphenyl (PCB) contaminants in insulating oil. Environ. Sci. Technol. 2014, 48, 8094–8100. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Naveas, N.; Degoutin, S.; Tabary, N.; Chai, F.; Spampinato, V.; Ceccone, G.; Rossi, F.; Torres-Costa, V.; Manso-Silvan, M.; et al. Porous silicon-cyclodextrin based polymer composites for drug delivery applications. Carbohyd. Polym. 2014, 110, 238–252. [Google Scholar] [CrossRef]
- Di Cagno, M.; Nielsen, T.T.; Lambertsen Larsen, K.; Kuntsche, J.; Bauer-Brandl, A. β-Cyclodextrin-dextran polymers for the solubilization of poorly soluble drugs. Int. J. Pharm. 2014, 468, 258–263. [Google Scholar]
- Zhang, Y.; Cai, X.; Xiong, W.; Jiang, H.; Zhao, H.; Yang, X.; Li, C.; Fu, Z.; Chen, J. Molecular insights into the pH-dependent adsorption and removal of ionizable antibiotic oxytetracycline by adsorbent cyclodextrin polymers. PLoS One 2014, 9, e86228. [Google Scholar]
- Wintgens, V.; Dalmas, F.; Sébille, B.; Amiel, C. Novel phosphorus-containing cyclodextrin polymers and their affinity for calcium cations and hydroxyapatite. Carbohyd. Polym. 2013, 98, 896–904. [Google Scholar] [CrossRef]
- Shirasawa, T.; Ueda, M.; Appell, M.; Goto, T. Use of cyclodextrin-based polymer for patulin analysis in apple juice. Mycotoxins 2013, 63, 1–8. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Sawy, S.M.; Ragaei, M.; Hamdy, I.A.; El-Bisi, M.K.; Abdel-Mohdy, F.A. New textiles of biocidal activity by introduce insecticide in cotton-poly (GMA) copolymer containing β-Cd. Carbohyd. Polym. 2014, 99, 208–217. [Google Scholar] [CrossRef]
- Simões, S.M.N.; Veiga, F.; Ribeiro, A.C.F.; Figueiras, A.R.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular gels of poly-α-cyclodextrin and PEO-based copolymers for controlled drug release. Eur. J. Pharm. Biopharm. 2014, 87, 579–588. [Google Scholar] [CrossRef]
- Olteanu, A.; Aramă, C.-C.; Radu, C.; Mihăescu, C.; Monciu, C.-M. Effect of β-cyclodextrins based nanosponges on the solubility of lipophilic pharmacological active substances (repaglinide). J. Incl. Phenom. Macrocycl. Chem. 2014, 1–8. [Google Scholar]
- Raoov, M.; Mohamad, S.; Abas, M. Synthesis and characterization of β-cyclodextrin functionalized ionic liquid polymer as a macroporous material for the removal of phenols and As(V). Int. J. Mol. Sci. 2013, 15, 100–119. [Google Scholar] [CrossRef]
- Gong, A.; Ping, W.; Wang, J.; Zhu, X. Cyclodextrin polymer/Fe3O4 nanocomposites as solid phase extraction material coupled with UV–vis spectrometry for the analysis of rutin. Spectrochim. Acta A 2014, 122, 331–336. [Google Scholar] [CrossRef]
- Ghemati, D.; Aliouche, D. Dye adsorption behavior of polyvinyl alcohol/glutaraldehyde/β-cyclodextrin polymer membranes. J. Appl. Spectrosc. 2014, 81, 257–263. [Google Scholar] [CrossRef]
- Bui, M.P.; Seo, S.S. Fabrication of polymerized crystalline colloidal array thin film modified β-cyclodextrin polymer for paraoxon-ethyl and parathion-ethyl detection. Anal. Sci. 2014, 30, 581–587. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Gong, X.; Diao, G. Universal water-soluble cyclodextrin polymer–carbon nanomaterials with supramolecular recognition. Carbon 2013, 61, 154–163. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects. Molecules 2014, 19, 14066-14079. https://doi.org/10.3390/molecules190914066
Folch-Cano C, Yazdani-Pedram M, Olea-Azar C. Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects. Molecules. 2014; 19(9):14066-14079. https://doi.org/10.3390/molecules190914066
Chicago/Turabian StyleFolch-Cano, Christian, Mehrdad Yazdani-Pedram, and Claudio Olea-Azar. 2014. "Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects" Molecules 19, no. 9: 14066-14079. https://doi.org/10.3390/molecules190914066