Photochemistry and Radical Chemistry under Low Intensity Visible Light Sources: Application to Photopolymerization Reactions
Abstract
:1. Introduction
2. Results and Discussion
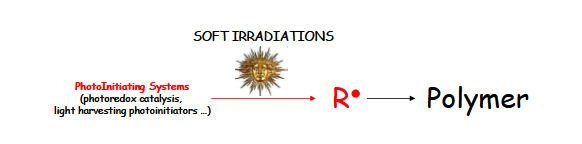
2.1. The Novel Strategy
- (i)
- high intensity light sources (e.g., a few W/cm² with a Hg lamp or focused laser beams) as the high amount of IS easily counterbalances the loss due to the oxygen quenching reactions;
- (ii)
- highly viscous media (viscosities > 1000 cp) where the diffusion rate constant kdiff and accordingly all bimolecular rate constants level off: this means that the oxygen quenching of radicals is therefore slowed down;
- (iii)
- thick samples; in thin samples, a very fast re-oxygenation is observed in the course of the photopolymerization, leading to a higher oxygen inhibition.







2.2. Performance of the Novel Photoinitiating Systems
2.3. Development of Light Harvesting Photoinitiators



2.4. Design of Systems Generating Silyl Radicals

2.5. Photoredox Catalysis in Photopolymerization Reactions Using Cheap and Non-Toxic Metal Complexes
(a) Copper and iron complexes as cheap or lower toxicity photocatalysts


(b) The photoredox catalysis using novel organophotocatalysts

3. Experimental Section
3.1. Steady State Photolysis Experiment
3.2. Redox Potentials
3.3. ESR Spin Trapping (ESR-ST) Experiment
3.4. Photopolymerization Experiments
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: New York, NY, USA, 1991. [Google Scholar]
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH.: Weinheim, Germany, 2012. [Google Scholar]
- Belfied, K.D.; Crivello, J.V. Photoinitiated Polymerization; ACS Symposium Series 847; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Davidson, S. Exploring the Science, Technology and Application of UV and EB Curing; Sita Technology Ltd.: London, UK, 1999. [Google Scholar]
- Neckers, D.C. UV and EB at the Millenium; Sita Technology: London, UK, 1999. [Google Scholar]
- Fouassier, J.P. Photoinitiation, Photopolymerization, Photocuring; Hanser: Münich, Germany, 1995. [Google Scholar]
- Photopolymerization: Fundamentals and Applications; Scranton, A.B.; Bowman, A.; Peiffer, R.W. (Eds.) ACS Symposium Series 673; American Chemical Society: Washington, DC, USA, 1997.
- Lasers in Polymer Science and Technology: Applications; Fouassier, J.P.; Rabek, J.F. (Eds.) CRC Press: Boca Raton, FL, USA, 1990.
- Pappas, S.P. UV-Curing: Science and Technology; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Radiation Curing in Polymer Science and Technology; Fouassier, J.P.; Rabek, J.F. Chapman & Hall: London, UK, 1993. [Google Scholar]
- Fouassier, J.P. Photochemistry and UV Curing; Fouassier, J.P., Ed.; Research Signpost: Trivandrum, India, 2006. [Google Scholar]
- Mishra, M.K.; Yagci, Y. Handbook of Vinyl Polymers; Mishra, M.K., Yagci, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Dietliker, K. A Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd.: Edinburgh, London, UK, 2002. [Google Scholar]
- Crivello, J.V. Photoinitiators for Free Radical, Cationic and Anionic Photopolymerization, 2nd ed.; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Nagib, D.A.; Scott, M.E.; MacMillan, D.W.C. Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2009, 131, 10875–10877. [Google Scholar] [CrossRef]
- Shih, H.-W.; Vander Wal, M.N.; Grange, R.L.; MacMillan, D.W.C. Enantioselective α-Benzylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2010, 132, 13600–13603. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Narayanam, J.M.R.; Stephenson, C.R.J. Visible-light-mediated conversion of alcohols to halides. Nat. Chem. 2011, 3, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Tucker, J.W.; Konieczynska, M.D.; Stephenson, C.R.J. Intermolecular Atom Transfer Radical Addition to Olefins Mediated by Oxidative Quenching of Photoredox Catalysts. J. Am. Chem. Soc. 2011, 133, 4160–4163. [Google Scholar] [CrossRef] [PubMed]
- Ischay, M.A.; Lu, Z.; Yoon, T.P. [2+2] Cycloadditions by Oxidative Visible Light Photocatalysis. J. Am. Chem. Soc. 2010, 132, 8572–8574. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yoon, T.P. Crossed Intermolecular [2+2] Cycloadditions of Acyclic Enones via Visible Light Photocatalysis. J. Am. Chem. Soc. 2009, 131, 14604–14605. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, M.H.; Pellet, R.; Fensterbank, L.; Goddard, J.P.; Lacôte, E.; Malacria, M.; Ollivier, C. Visible-Light-Induced Photoreductive Generation of Radicals from Epoxides and Aziridines. Angew. Chem. Int. Ed. 2011, 50, 4463–4466. [Google Scholar] [CrossRef]
- Courant, T.; Masson, G. Photoredox-Initiated α-Alkylation of Imines through a Three-Component Radical/Cationic Reaction. Chem. Eur. J. 2012, 18, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Baralle, A.; Fensterbank, L.; Goddard, J.P.; Ollivier, C. Aryl Radical Formation by Copper(I) Photocatalyzed Reduction of Diaryliodonium Salts: NMR Evidence for a CuII/CuI Mechanism. Chem. Eur. J. 2013, 19, 10809–10813. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Fuldner, S.; Konig, B.; Zeitler, K. Metal-Free, Cooperative Asymmetric Organophotoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2011, 50, 951–954. [Google Scholar] [CrossRef]
- Zeitler, K. Photoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2009, 48, 9785–9789. [Google Scholar] [CrossRef]
- Lalevée, J.; Dirani, A.; El-Roz, M.; Allonas, X.; Fouassier, J.P. Silanes as New Highly Efficient Co-initiators for Radical Polymerization in Aerated Media. Macromolecules 2008, 41, 2003–2010. [Google Scholar] [CrossRef]
- Lalevee, J.; Fouassier, J.P. Overview of Radical Initiation, Tome 1—Chapter 2 in Encyclopedia of Radicals in Chemistry. In Biology & Materials; Studer, A., Chatgililoglu, C., Eds.; Wiley: Weinheim, Germany, 2012. [Google Scholar]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Metal and metal-free photocatalysts: mechanistic approach and application as photoinitiators of photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.A.; Lalevée, J.; Fouassier, J.P. A breakthrough toward long wavelength cationic photopolymerization: Initiating systems based on violanthrone derivatives and silyl radicals. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Julolidine or Fluorenone Based Push-Pull Dyes for Polymerization upon Soft Polychromatic Visible Light or Green Light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Green Bulb Light Source Induced Epoxy Cationic Polymerization under Air Using Tris(2,2'-bipyridine)ruthenium(II) and Silyl Radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A Novel Photopolymerization Initiating System Based on an Iridium Complex Photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient dual radical/cationic photoinitiator under visible light: a new concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. Household LED irradiation under air: cationic polymerization using iridium or ruthenium complex photocatalysts. Polym. Bull. 2012, 68, 341–347. [Google Scholar] [CrossRef]
- Lalevée, J.; Peter, M.; Dumur, F.; Gigmes, D.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Subtle Ligand Effects in Oxidative Photocatalysis with Iridium Complexes: Application to Photopolymerization. Chem. Eur. J. 2011, 17, 15027–15031. [Google Scholar] [PubMed]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Dumur, F.; Gigmes, D.; Blanchard, N.; Fouassier, J.P. Photoredox catalysis for polymerization reactions. Chimia 2012, 66, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Photopolymerization of N-Vinylcarbazole Using Visible-Light Harvesting Iridium Complexes as Photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.P. Iridium Photocatalysts in Free Radical Photopolymerization under Visible Lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. Recent advances in sunlight induced polymerization: Role of new photoinitiating systems based on the silyl radical chemistry. Polym. Chem. 2011, 2, 1107–1113. [Google Scholar] [CrossRef]
- Telitel, S.; Lalevee, J.; Blanchard, N.; Kavalli, T.; Tehfe, M.A.; Schweitzer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Photopolymerization of cationic monomers and acrylate/divinylether blends under visible light using pyrromethene dyes. Macromolecules 2012, 45, 6864–6868. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N. Thioxanthone-ethyl anthracene. J. Photochem. Photobiol. A Chem. 2013, 257, 54–59. [Google Scholar] [CrossRef]
- Doğruyol, S.K.; Doğruyol, Z.; Arsu, N. Thioxanthone based 9-[2-(methyl-phenyl-amino)-acetyl]-thia-naphthacene-12-one as a visible photoinitiator. J. Lumin. 2013, 138, 98–104. [Google Scholar] [CrossRef]
- Corakci, B.; Hacioglu, S.O.; Toppare, L.; Bulut, U. Long wavelength photosensitizers in photoinitiated cationic polymerization: The effect of quinoxaline derivatives on photopolymerization. Polymer 2013, 54, 3182–3187. [Google Scholar] [CrossRef]
- Podsiadły, R.; Strzelczyk, R. N-substituted quinoxalinobenzothiazine/iodonium salt systems as visible photoinitiators for hybrid polymerization. Dyes Pigments 2013, 97, 462–468. [Google Scholar] [CrossRef]
- Shen, K.; Li, Y.; Liu, G.; Li, Y.; Zhang, X. Synthesis and photolytic properties of 1,5-di-N,N'-dialkylaminoanthraquinones containing acryloyl groups. Prog. Org. Coat. 2013, 76, 125–130. [Google Scholar] [CrossRef]
- Yang, J.; Tang, R.; Shi, S.; Nie, J. Synthesis and characterization of polymerizable one-component photoinitiator based on sesamol. Photochem. Photobiol. Sci. 2013, 12, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [PubMed]
- Bojinov, V.B.; Simeonov, D.B. Synthesis of highly photostable blue-emitting 1,8-naphthalimides and their acrylonitrile copolymers. Polym. Degrad. Stab. 2010, 95, 43–52. [Google Scholar] [CrossRef]
- Noppakundilograt, S.; Suzuki, S.; Urano, T.; Miyagawa, M.; Takahara, S.; Yamaoka, T. Vis-sensitive photopolymer containing vinyl ether compound and pyrromethene dye. Polym. Adv. Technol. 2002, 13, 527–533. [Google Scholar] [CrossRef]
- Coenjarts, C.; Garcıa, O.; Llauger, L.; Palfreyman, J.; Vinette, A.L.; Scaiano, J.C. Mapping photogenerated radicals in thin polymer films: Fluorescence imaging using a prefluorescent radical probe. J. Am. Chem. Soc. 2003, 125, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Bojinov, V.; Grabchev, I. Synthesis of new polymerizable 1,8-naphthalimide dyes containing a 2-hydroxyphenylbenzotriazole fragment. Dyes Pigments 2003, 59, 277–283. [Google Scholar] [CrossRef]
- Grabchev, I.; Philipova, T. Copolymerization of acrylonitrile with some monomeric 1,8-naphthalimide fluorescent brighteners. Des. Monomers Polym. 2000, 3, 479–488. [Google Scholar] [CrossRef]
- Sharifi, S.; Mirzadeh, H.; Imani, M.; Ziaee, F.; Tajabadi, M.; Jamshidi, A.; Atai, M. Synthesis, photocrosslinking characteristics, and biocompatibility evaluation of N-vinyl pyrrolidone/polycaprolactone fumarate biomaterials using a new proton scavenger. Polym. Adv. Technol. 2008, 19, 1828–1838. [Google Scholar] [CrossRef]
- Lalevee, J.; Tehfe, M.A.; Dumur, F.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P. Light-Harvesting Organic Photoinitiators of Polymerization. Macromol. Rapid Commun. 2013, 34, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Copper Complexes in Radical Photoinitiating Systems: Applications to Free Radical and Cationic Polymerization upon Visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lalevée, J.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Fouassier, J.-P. Photochemistry and Radical Chemistry under Low Intensity Visible Light Sources: Application to Photopolymerization Reactions. Molecules 2014, 19, 15026-15041. https://doi.org/10.3390/molecules190915026
Lalevée J, Morlet-Savary F, Dietlin C, Graff B, Fouassier J-P. Photochemistry and Radical Chemistry under Low Intensity Visible Light Sources: Application to Photopolymerization Reactions. Molecules. 2014; 19(9):15026-15041. https://doi.org/10.3390/molecules190915026
Chicago/Turabian StyleLalevée, Jacques, Fabrice Morlet-Savary, Céline Dietlin, Bernadette Graff, and Jean-Pierre Fouassier. 2014. "Photochemistry and Radical Chemistry under Low Intensity Visible Light Sources: Application to Photopolymerization Reactions" Molecules 19, no. 9: 15026-15041. https://doi.org/10.3390/molecules190915026