Validation of reaction sequences
The objective of our validation study, by determining the scope and limitation of each reaction in the scheme, was to reach the necessary reliability level to obtain quality prod- ucts in the absence of purification steps, and without the pros- pect of having to characterize each library component with individual analyses. At times, rather complex mixtures of over 100 components per sublibrary arise from split-and-mix protocols. Our intention was to utilize the approach for broad lead finding with combinatorial libraries of original, semi- rigid molecules built-up from simple, commercially avail- able building blocks. The quality standards of complicated synthetic mixtures are critically dependent on the diligence of the previously run ‘chemical rehearsals’.
We handled the process analysis in two different steps: validation of all chemical steps by model syntheses of par-
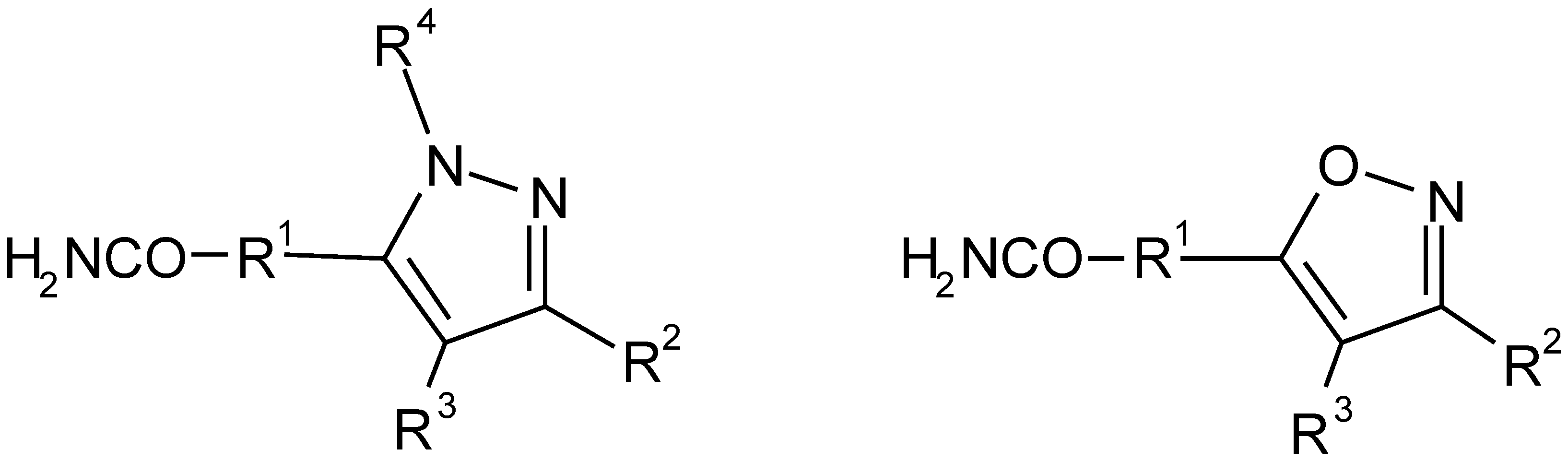
Scheme 1.
Sequence of reactions generating molecular diversity.
Scheme 1.
Sequence of reactions generating molecular diversity.
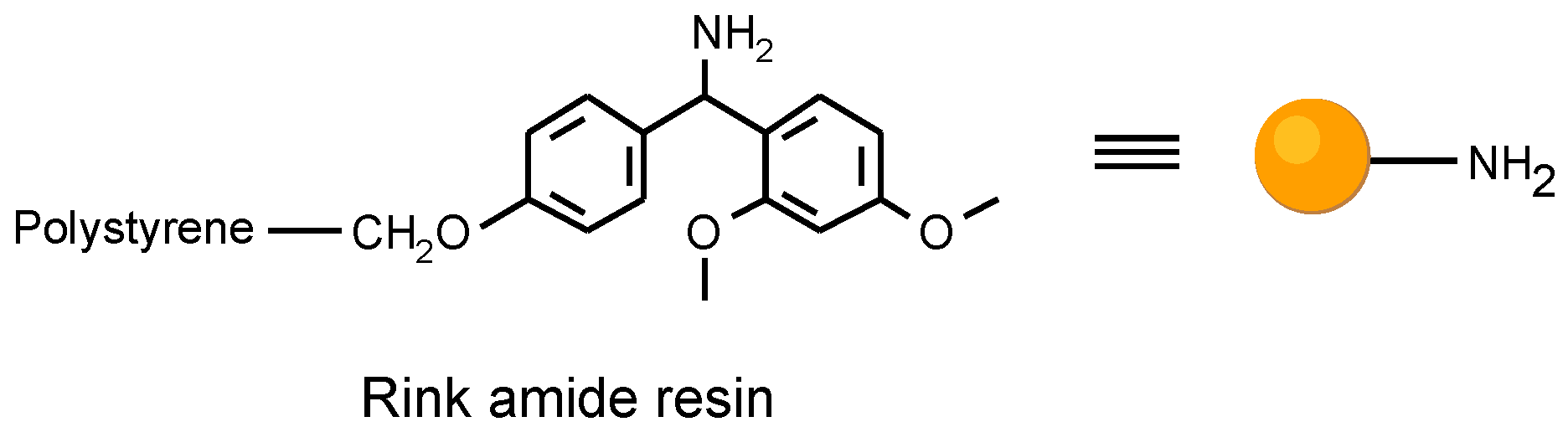
Figure 2.
The Rink amide resin utilized as solid support.
Figure 2.
The Rink amide resin utilized as solid support.
ticular compounds (with building blocks that are representa- tive of larger classes of analogs), followed by spectroscopic analysis of a number of library mixtures. In practice, after each transformation that was part of the validation on the solid support, the reaction products were cleaved, identified by MS, and the purity was defined by HPLC. To that end,
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5 list the residues of the reagents and products, of which the conversions on solid phase were monitored by LCMS of starting materials, as well as product samples, cleaved from the solid support into solution. Occasionally, compounds were prepared on a larger scale and were char- acterized by NMR, after cleavage from the resin. The capa- bility for high resolution structural elucidations of com- pounds, while covalently attached to the solid support, was reported in the literature [
4,
5] but not available to us in time for this study.
In planning the approach, we had to consider the resin matrix and the linkage to the solid support for the organic solid phase synthesis. The attachment to the solid support had to be compatible with the reaction sequence, and cleav- able after the template synthesis. We utilized a common poly- styrene resin with a trialkoxybenzhydryl linker (Rink amide resin) [
6].
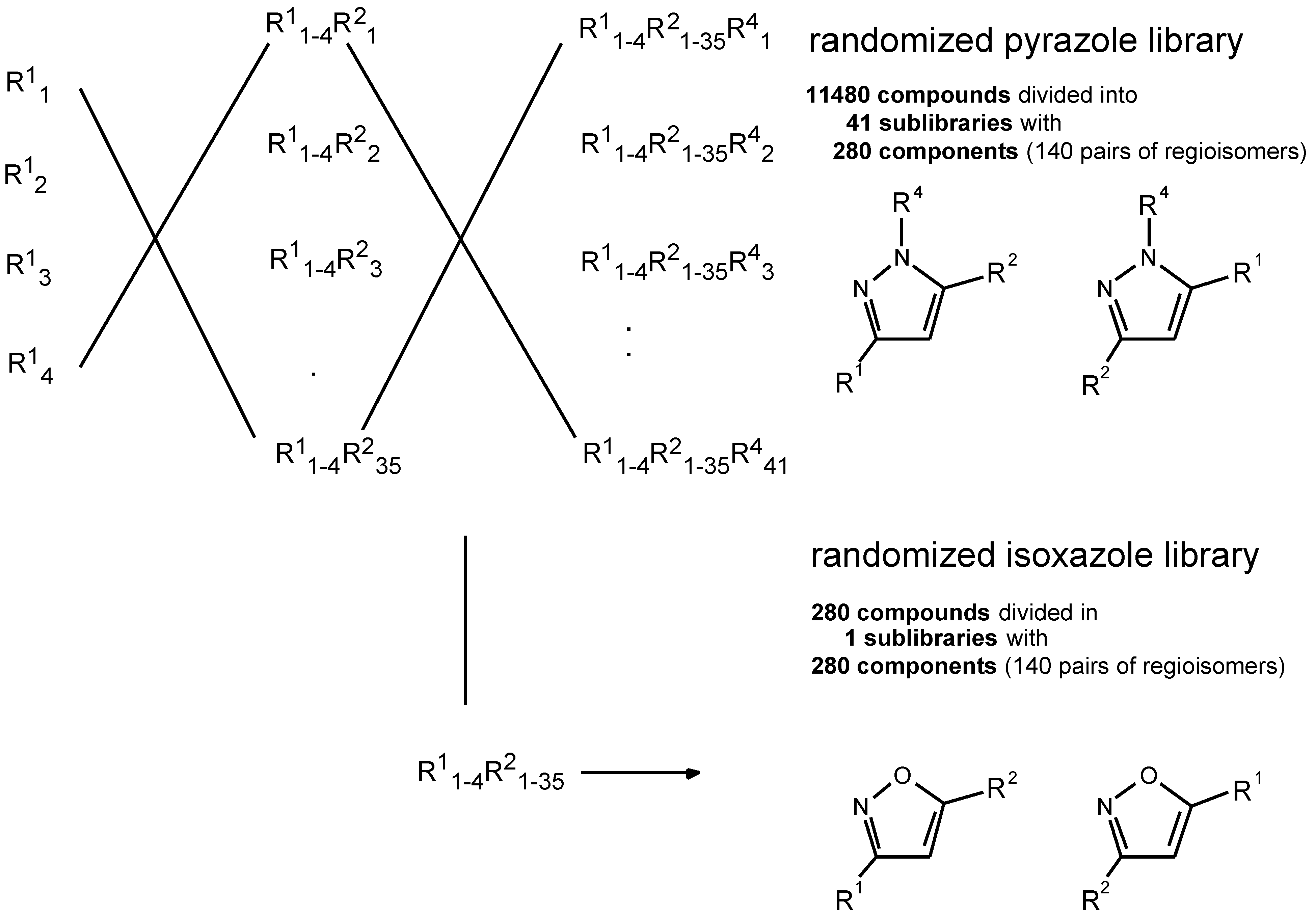
The fully substituted pyrazole scaffold is built from four different building block reagent classes, namely from car- boxylic acids with an acetyl function, carboxylic acid esters, alkylating agents, and monosubstituted hydrazines. A four step procedure with four different reagents leads to functionalities R1, R2, R3, and R4. However, the formation of regioisomers in the last reaction doubles the number of mol- ecules in a library.
In the first validation step the solid support was loaded with the R
1 component bearing the acetyl function. We ob- served quantitative transformation, after one hour, for vari- ous heteroaromatic derivatives
1a,
1b,
1c, and
1e, unless ortho substituted bifunctional derivatives like o-acetophenone and acetylphthalanilidic acid (
1d) were used (
Table 1), which undergo ring closure side reactions [
7]. After each transfor- mation the product was cleaved by treatment with 95% TFA and the quality was checked by HPLC (
Table 1). The purity of each component was higher than 95 % throughout. Con- sidering that traces of impurity were caused by partial break- down products of the linker, it can be assumed that the com- pounds on the resin were of excellent quality.
For the Claisen condensation, optimization of the reac- tion protocol with the prototypic aromatic ester ethyl ben- zoate ( 2a ) led to conditions, which also ensure that desactivated benzoates, e.g. 2b, 2h and heteroaromatic car- boxylic esters 2i-k with widely differing electronic proper- ties condense without appreciable formation of side prod- ucts. As expected, carboxylic esters with α-hydrogens (2g) are unsuited. Since there are more than one hundred mono- substituted aromatic carboxylic acid esters commercially available, this limitation is acceptable, until a strategy suit- able to incorporate aliphatic residues is worked out. Intrigu- ingly, the employed reaction conditions gave rise to partial reduction of a nitroaromatic building block (2c). Also weakly acidic heteroaromatic compounds cannot be applied. The ethyl indole carboxylate (2k) forms the indolyl anion, which lacks sufficient electrophilicity. Noticeably, the series of suc- cessful conversions to the diketone included a bifunctional building block and a component with an additional electrophilic center (2e and 2f). The reactivity of further aro- matic carboxylic esters could be estimated by the Hammett equation.
In general, β-diketones undergo α-alkylationin the pres- ence of the phase transfer catalyst tetra-
n-butylammonium
Table 1.
Building blocks used for R1 validation and the corresponding values of loading capacity ob- tained on the resin.
Table 1.
Building blocks used for R1 validation and the corresponding values of loading capacity ob- tained on the resin.
Table 2.
Claisen condensation to β-diketone 2a-l (R1= 4-Ph).
Table 2.
Claisen condensation to β-diketone 2a-l (R1= 4-Ph).
hydrogen sulfate in water/methylene chloride. Substrate
2a was examined under these conditions. We used NaOH as the base and EtI as alkylating agent. After 16h at room temperature no starting material was detected. Although α-alkylation was the major product, we identified O-alkylation and various side products.
For the α-alkylation step, the best results were obtained in the presence of TBAF, which shields the oxygen atoms of the β-dicarbonyl intermediate by complexation, hence inhibiting O-alkylation as a side reaction and furthermore increasing the nucleophilicity of the compound. Under strictly anhydrous conditions, yields of 90% in C-monoalkylated product can be obtained. To expand the diversity, we also tested alkylating agents other than the simple alkyl iodides described in analogous solution chemistry [
8]. Ethyl bromoacetate (
3c) and allyl bromide (
3f) reacted without side reactions. The failure with benzyl bromide (
3b) was rather unexpected. Iodoacetonitrile (
3d) and bromoacetophenone (
3e) were unsatisfactory.
A large number of nitrogen containing heteroaromatic
Table 3.
aα-Alkylation of diketone 2a (R1 = 4-Ph, R2 = Ph).
Table 3.
aα-Alkylation of diketone 2a (R1 = 4-Ph, R2 = Ph).
compounds are commercially available for the R
2 position in the previous Claisen condensation. Various side products could arise upon alkylation of these compounds. Experimentally, we confirmed that the alkylation step is incompatible with the presence of acidic or basic heteroaromatic R
1 and R
2 residues: with the phenyl pyridine diketone
2k several side products were observed upon alkylation with the alkylating agents
3a,
3c,
3d and
3f. Therefore, for the preparation of combinatorial libraries, a strategy that skips the alkylation step enables a broader choice of building blocks for the previous Claisen condensation, by allowing e.g. the inclusion of N-heterocyclic esters as an alternative source of diversity.
The ring closure to form a heterocyclic scaffold progressed quantitatively with hydrazines 4a-c and with hydroxylamine (5a).
For further validation the reaction of structurally and electronically diverse substrates were synthesized on a larger scale, so that these compounds could be isolated and identified by NMR.
Compound
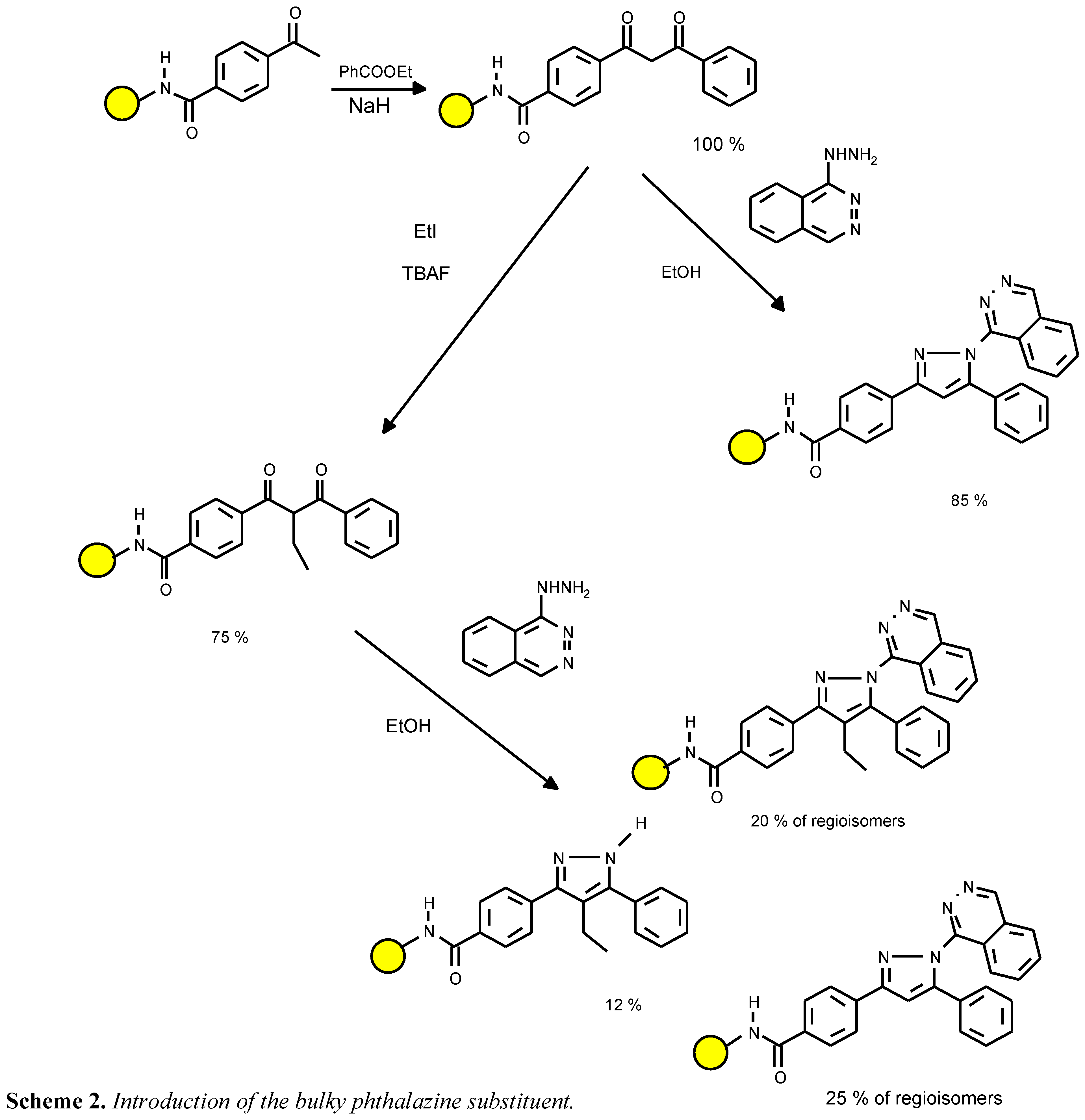
4d was prepared from hydralazine with the intention of exploring the limits of using building blocks with unfavourable electronic and steric properties (see
Scheme 2). The reaction was sluggish and after one day, only traces of both regioisomers
4d were detectable. A 20% conversion yield was reached after four days. The presence of the unsubstituted analog
4a indicated that the non-alkylated diketone precursor ( a residual impurity) is far more reactive than the alkylated intermediate.
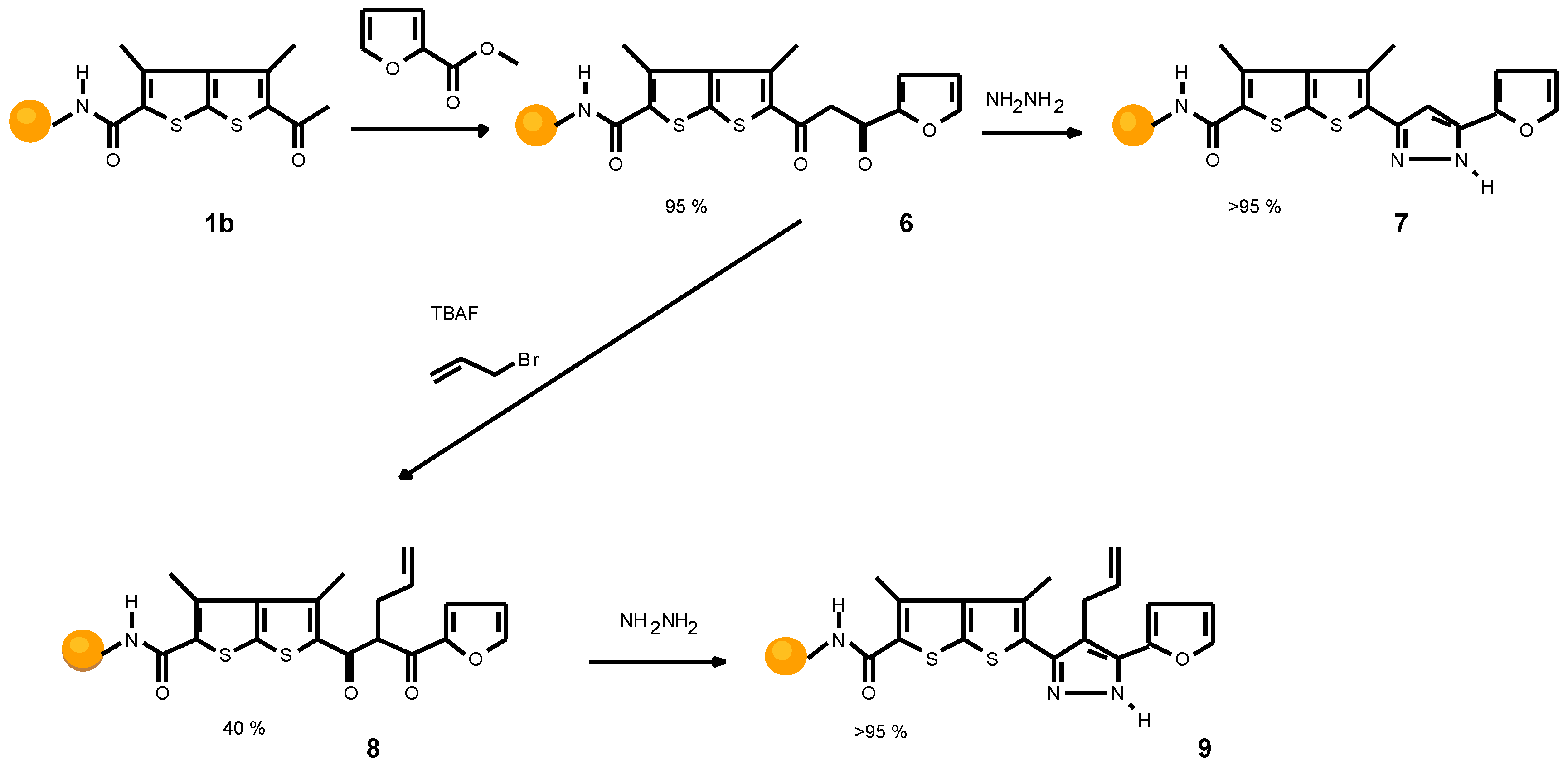
Scheme 3 outlines the synthesis of a furyl pyrazole (
9). A quantitative transformation was observed for the coupling procedure and the following Claisen condensation which af- fords diketone
6. Unfortunately, alkylation of
6 leads to the alkylated diketone only in 40% yield. Bis-alkylated prod- ucts and also a small amount of starting material were iden- tified. In order to isolate and characterize the final product, hydrazine was chosen as cyclization reagent, to avoid the formation of regioisomers. Compound
9 was formed with- out side products.
The difficulties in predicting the influence of different substituents on the alkylation together with the broad devia- tions in yield prompted us to dispense with the alkylation for the preparation of a first, diverse library.
Our strategy was then to study other reagents which could be used in the cyclization reaction of non-alkylated diketones. We found (
Table 5) that the twofold nucleophilic character of monosubstituted hydrazines, including desactivated hydrazines, and also hydroxyl amine, which leads to isoxazoles, allows them to react with each carbonyl group of a 1,3-diketone in satisfactory yield. It was found that the con- version of reactive hydrazines, like methyl hydrazine (
4f) or benzylhydrazine (
4h), is nearly quantitative, which means that there is no (or only a trace of) starting material and no impurities caused by the cyclization reaction. Also for aro- matic hydrazines with strong electron withdrawing groups,
Scheme 2.
Introduction of the bulky phthalazine substituent.
Scheme 2.
Introduction of the bulky phthalazine substituent.
Table 4.
Formation of pyrazoles 4a-4d (R1 = 4-Ph, R2 = Ph, R3 = Et) and isoxazole 5a (R1 = 4-Ph, R2 = Ph, R3 = Et).
Table 4.
Formation of pyrazoles 4a-4d (R1 = 4-Ph, R2 = Ph, R3 = Et) and isoxazole 5a (R1 = 4-Ph, R2 = Ph, R3 = Et).
Scheme 3.
Synthesis of furyl pyrazole 9.
Scheme 3.
Synthesis of furyl pyrazole 9.
like pyrimidines or even phthalazines, we observed a satis- factory transformation. As expected, acid hydrazides like
4i are unsuited. The major product of this reagent is the N- unsubstituted pyrazole
4e, because the 1-acylpyrazoles are cleaved by nucleophilic attack.
In the course of our investigations we found that, for rea- gents with low solubilities, saturated solutions are often sufficent to drive the reaction to completion. As many rea- gents as possible, particularly those with questionable reac- tivity (e.g. for steric or electronic reasons), should be tested in model reactions before they are positively selected for a library preparation, so as to minimize unpredictable results.
Table 5.
Formation of pyrazoles 4e-n (R1 = 4-Ph, R2 = Ph, R3 = H).
Table 5.
Formation of pyrazoles 4e-n (R1 = 4-Ph, R2 = Ph, R3 = H).
For example it was found that some aromatic hydrazines contain the far more reactive hydrazine as an impurity. With a 5% contamination and usage of a 20-fold excess of the actual reagent, the reaction product obtained is almost ex- clusively the N-unsubstituted pyrazole. We found that the addition of acetylacetone is an excellent scavenger for hydrazine impurities. Another reason to examine the reac- tivity of reagents experimentally is to eliminate those rea- gents, which are not soluble or which decompose under the specific reaction conditions. It is known that hydrazines tend to decompose and loose nitrogen if kept at high temperature for prolonged periods of time.
The collected data on the scope and limitations of the various chemical reactions, that are part of the envisaged scheme for molecular diversity generation, provided the nec- essary information for a diligent planning of combinatorial libraries according to the
split-and-mix principle [
2]. The preparation of a first library with more than 10,000 compo- nents is described in the following sections.