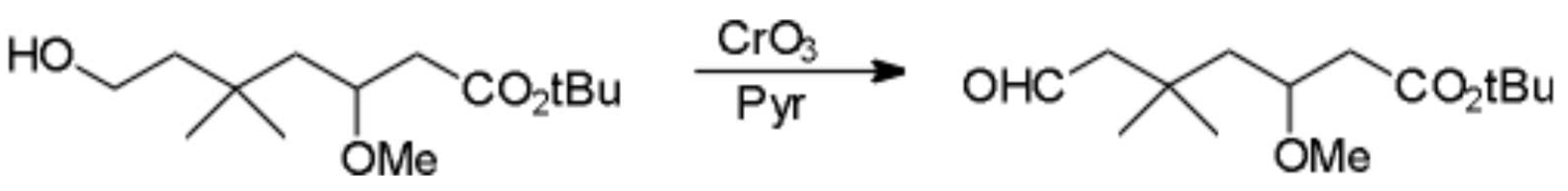
tert-Butyl-3-methoxy-5,5-dimethyl-7-oxo-heptanoate
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Smith, D. tert-Butyl-3-methoxy-5,5-dimethyl-6-heptenoate. Molecules 1997, 2, M30. [Google Scholar] [CrossRef]
- Smith, D. tert-Butyl-3-methoxy-5,5-dimethyl-7-hydroxy-heptanoate. Molecules 1997, 2, M31. [Google Scholar] [CrossRef]
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved
Share and Cite
Smith, D.A. tert-Butyl-3-methoxy-5,5-dimethyl-7-oxo-heptanoate. Molecules 1997, 2, M32. https://doi.org/10.3390/M32
Smith DA. tert-Butyl-3-methoxy-5,5-dimethyl-7-oxo-heptanoate. Molecules. 1997; 2(10):M32. https://doi.org/10.3390/M32
Chicago/Turabian StyleSmith, Douglas A. 1997. "tert-Butyl-3-methoxy-5,5-dimethyl-7-oxo-heptanoate" Molecules 2, no. 10: M32. https://doi.org/10.3390/M32



