Synthesis of 1,2,3,4,5,6-Hexahydrophosphinine 1-Oxides by Catalytic Hydrogenation of 3-Phosphabicyclo[3.1.0]hexane 3-Oxides
Abstract
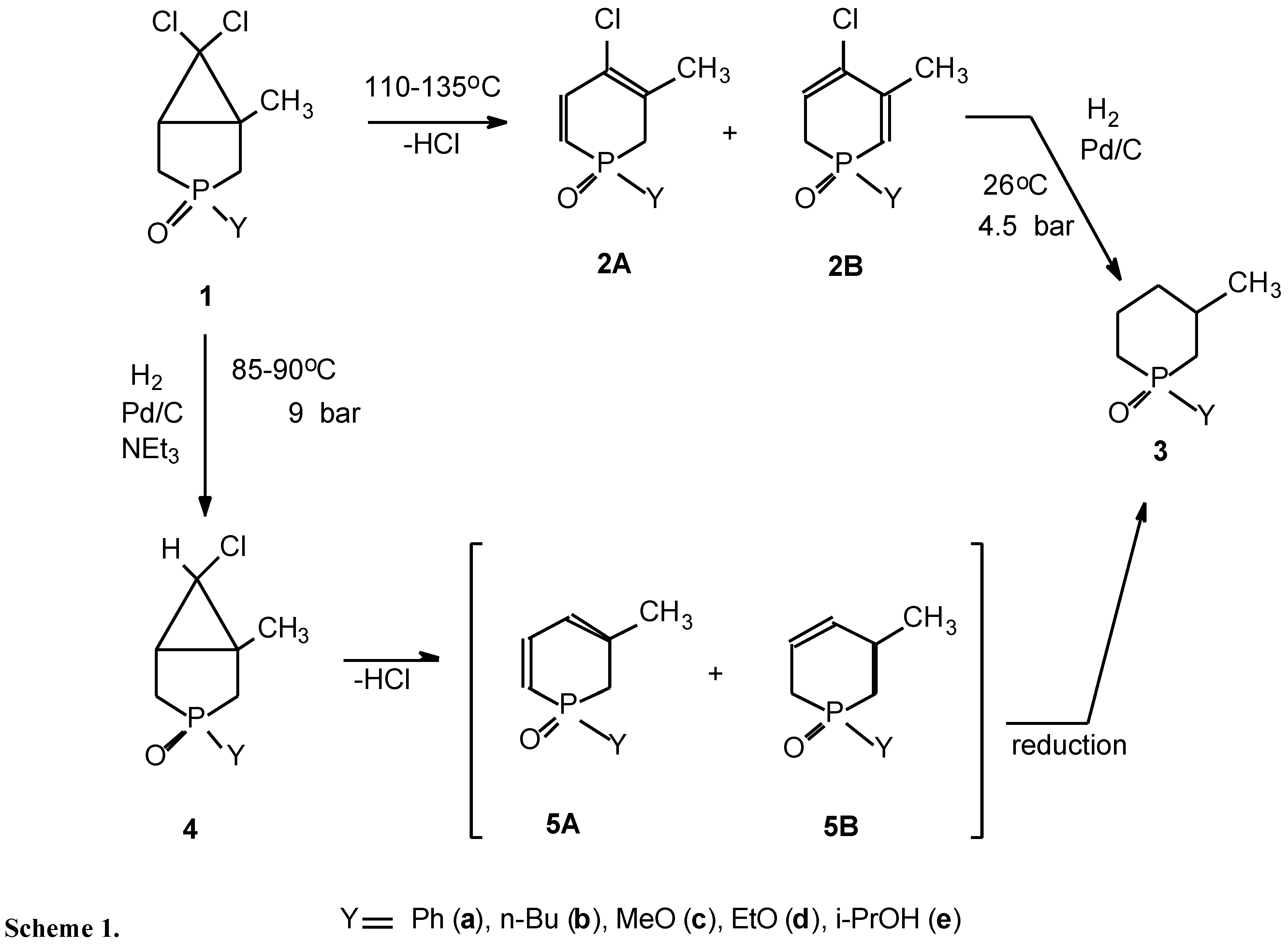
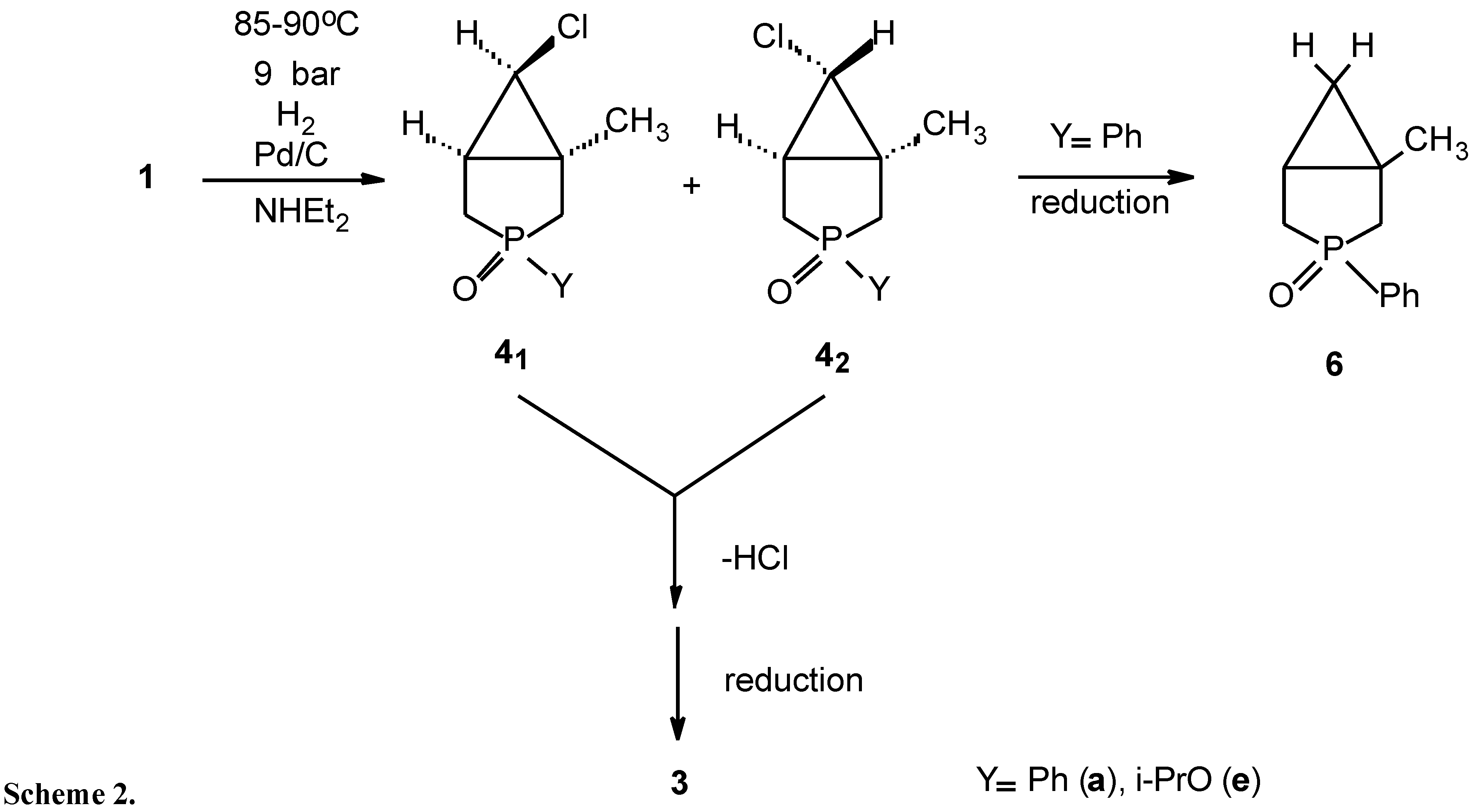
:| Y | 1→2→3 | 1→4→5→3 |
|---|---|---|
| Yield (%) | ||
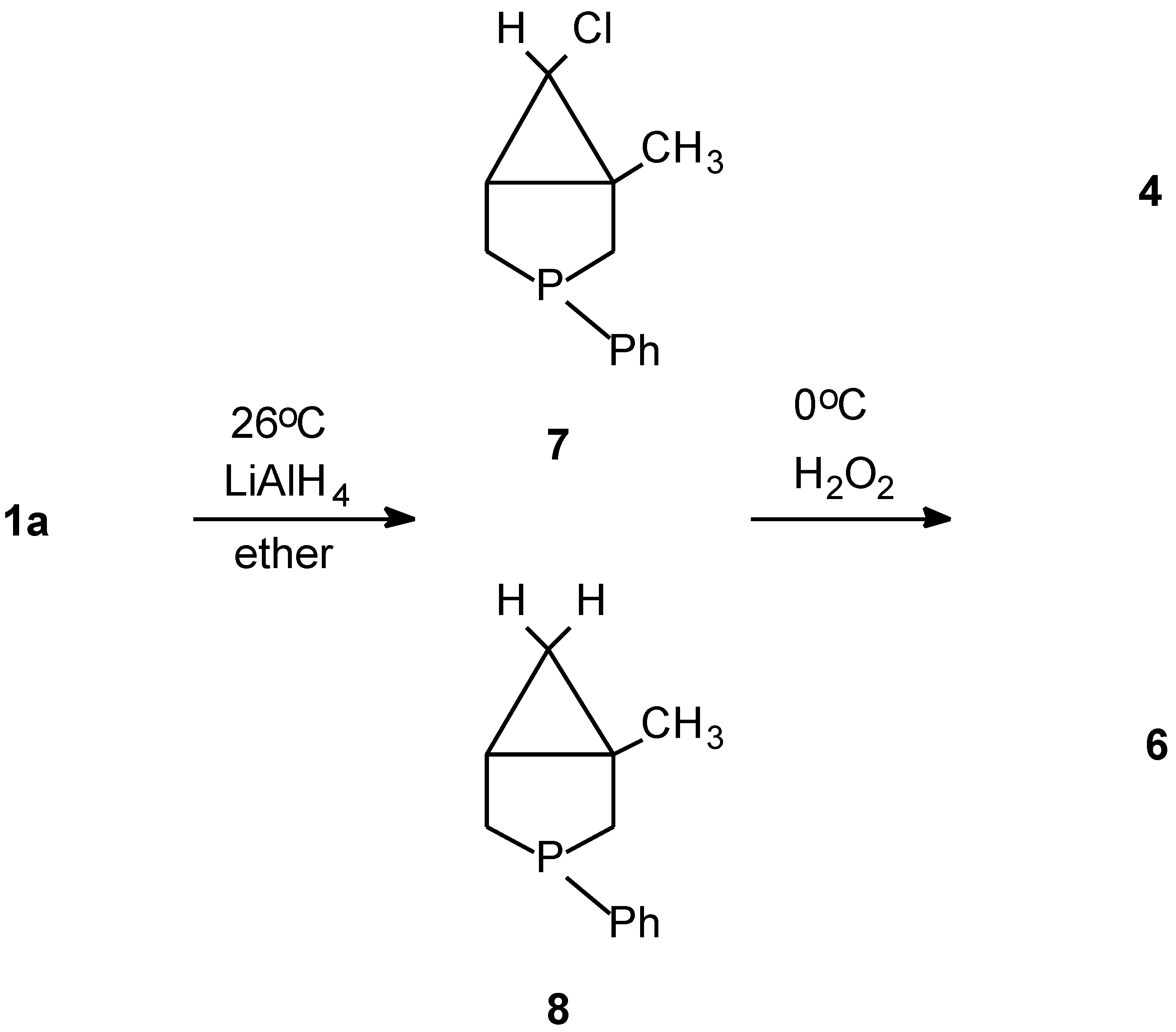
| Ph (a) | - | 70 |
| n-Bu (b) | - | 58 |
| EtO (c) | 25 | 73 |
| n-PrO (d) | 28 | 60 |
| i-PrO (e) | 40 | 78 |
Acknowledgement
References
- Keglevich, Gy.; Petneházy, I.; Miklós, P.; Almásy, A.; Tóth, G.; Tôke, L.; Quin, L. D. J. Org. Chem. 1987, 52, 3983. [CrossRef]
- Keglevich, Gy.; Brlik, J.; Janke, F.; Tôke, L. Heteroatom Chem. 1990, 1, 419.
- Keglevich, Gy.; Kovács, A.; Újszászy, K.; Tungler, A.; Tóth, G.; Tôke, L. Phosphorus Sulfur 1992, 70, 219.
- Keglevich, Gy.; Tungler, A.; Novák, T.; Tôke, L. J. Chem. Res. (S) 1996, 528.
- Sample Availability: Supporting sample 3a, MDPI 4683, is available from MDPI.
© 1997 MDPI. All rights reserved
Share and Cite
Keglevich, G.; Tungler, A.; Újszászy, K.; Novák, T.; Tôke, L. Synthesis of 1,2,3,4,5,6-Hexahydrophosphinine 1-Oxides by Catalytic Hydrogenation of 3-Phosphabicyclo[3.1.0]hexane 3-Oxides. Molecules 1997, 2, 43-45. https://doi.org/10.3390/feb97p3
Keglevich G, Tungler A, Újszászy K, Novák T, Tôke L. Synthesis of 1,2,3,4,5,6-Hexahydrophosphinine 1-Oxides by Catalytic Hydrogenation of 3-Phosphabicyclo[3.1.0]hexane 3-Oxides. Molecules. 1997; 2(2):43-45. https://doi.org/10.3390/feb97p3
Chicago/Turabian StyleKeglevich, György, Antal Tungler, Kálmán Újszászy, Tibor Novák, and László Tôke. 1997. "Synthesis of 1,2,3,4,5,6-Hexahydrophosphinine 1-Oxides by Catalytic Hydrogenation of 3-Phosphabicyclo[3.1.0]hexane 3-Oxides" Molecules 2, no. 2: 43-45. https://doi.org/10.3390/feb97p3




