Experimental
General
1H NMR spectra were recorded at 300 MHz on a Varian VXR 300 or at 80 MHz on a Tesla BS 487 at 293 K in CDCl3. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS. Multiplicities are reported as singlet (s), doublet (d), triplet (t), quartet (q), some combination of these, broad (br), or multiplet (m). 13C NMR spectra were recovered at 75.0 MHz on the same spectrometer as 1H NMR spectra at 293 K in CDCl3. NMR analysis of the crude original mixture permitted a determination of ratio of the diastereoisomers. Flash chromatography was carried out on 63-200 μm or 40-60 μm silica gel. Thin layer chromatography was carried out on aluminium backed silica plates containing UV254 by Lachema and plates were visualized with UV light and Mostaine solution as appropriate. All yields refer to isolated, spectroscopically pure material, and have not been optimized.
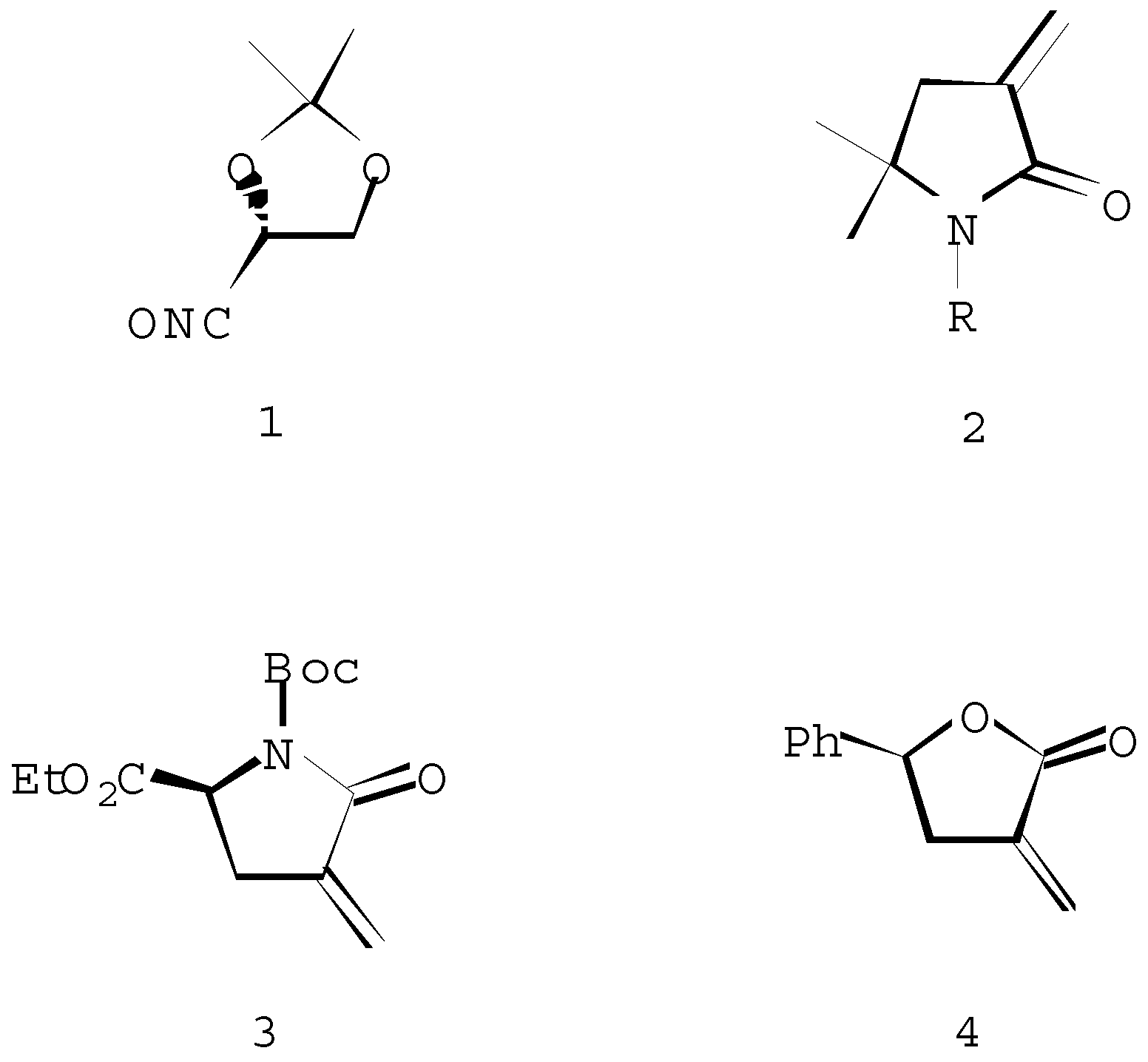
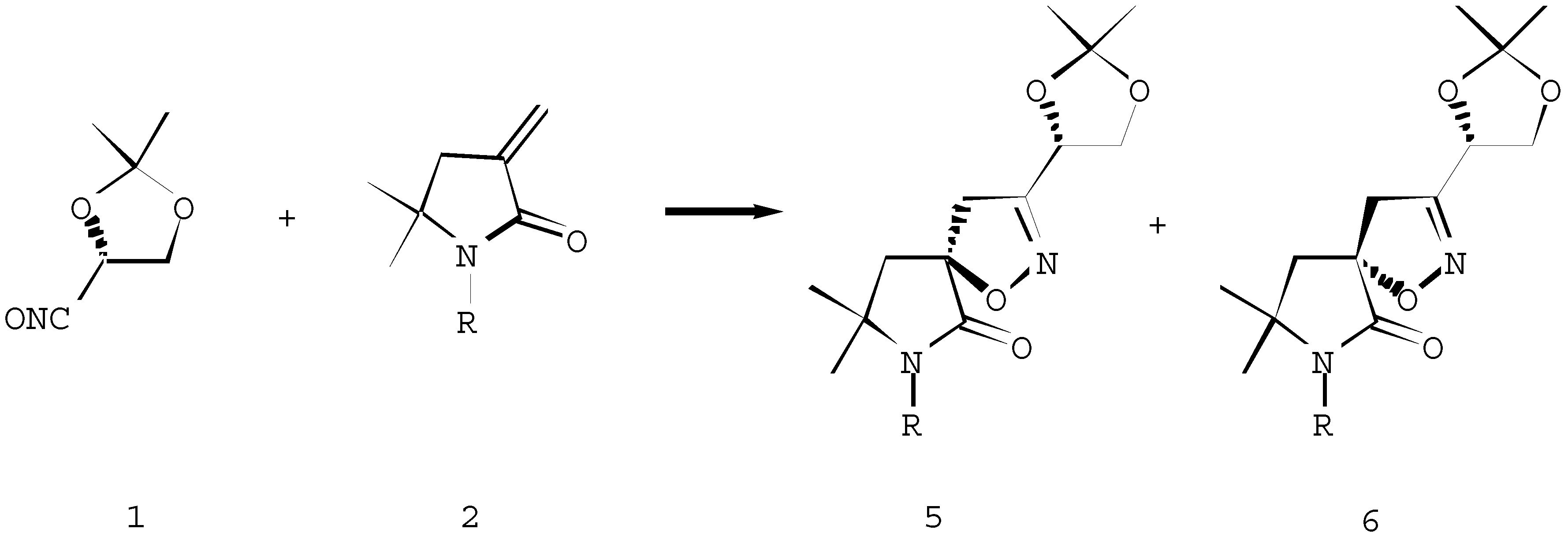
Spiroisoxazolines 5a-e, 6a-e. General procedure
A solution of Et3N (8.8 mmol) in chloroform (25 ml) was added during 24 hours to a stirred solution of the dipolarophile 2 (8.0 mmol) and the unstable nitrile oxide 1 (8.0 mmol, prepared in situ from the corresponding hydroxymoyl chloride) in chloroform (25 ml). After evaporation of the solvent, the products were isolated as an inseparable mixture of diastereoisomers 5 and 6.
(1´S, 5R/S) 8,8-Dimethyl-3-(1´,2´-di-O-isopropylidene-1´,2´-dihydroxyethyl)-6-oxo-1-oxa-2,7-diazaspiro[4,4]- non-2-ene (5a + 6a)
1H NMR (CDCl3, 300 MHz): 5.0-5.3 (1H, m), 4.1-4.5 (2H, m), 2.0-3.8 (4H, m), 1.4, 1.5, 1.7, 1.8, 1.9 (12H, s, Me). 13C-NMR (CDCl3, 300 MHz): 171.66, 171.60, 156.49, 156.41 (C, 4-C, 9-C), 111.17, 110.93 (C, OCO), 87.17, 86.40 (C, 5-C), 69.78, 69.73 (CH, 1´-C), 65.80, 65.73, 53.13 (C, CH2, 8-C, 2´-C), 47.52, 47.26, 40.58, 40.44 (CH2, 4-C, 9-C), 28.30, 28.26, 25.12, 25.08, 24.95, 24.37, 24.08, 24.00 (CH3, Me).
(1´S, 5R/S) 7-Butyl-8,8-dimethyl-3-(1´,2´-di-O-isopropy-lidene-1´,2´-dihydroxyethyl)-6-oxo-1-oxa-2,7-diazaspiro-[4,4]non-2-ene (5b + 6b)
1H-NMR (CDCl3, 300 MHz): 4.5-4.9 (3H, m), 3.8-3.9 (2H, m), 1.3-3.3 (20H, m), 0.9-1.0 (3H, m). 13C-NMR (CDCl3, 300 MHz): 170.31, 158.74, 158.63 (C, 4-C, 9-C), 113.84 (C, OCO), 85.37 (C, 5-C), 72.58, 72.56 (CH, 1´-C), 62.69, 63.40 (CH2, 2´-C), 57.60, 57.49 (C, 8-C), 46.88, 46.66, 45.57, 42.07, 41.68, 40.44, 38.93, 38.7, 19.37 (CH2, CH2), 30.55, 30.25, 28.76, 26.73, 26.58, 26.39, 12.71 (CH3, Me).
(1´S, 5R/S) 7-Acetyl-8,8-dimethyl-3-(1´,2´-di-O-isopropy-lidene-1´,2´-dihydroxyethyl) -6-oxo-1-oxa-2,7-diazaspiro-[4,4]non-2-ene (5c + 6c)
1H-NMR (CDCl3, 300 MHz): 3.5-4.9 (5H, m), 3.1-3.3 (2H, m), 2.5 (3H, s), 2.0-2.4 (2H, m), 1.4, 1.5, 1.6 (12H, s, Me). 13C-NMR (CDCl3, 300 MHz): 172.88, 172.43, 171.26. 171.20, 157.31, 157.09 (C, 6-C, 3-C, N-CO), 109.83, 109.76 (C, OCO), 85.86, 85.78 (C, 5-C), 70.12, 70.04 (CH, 1´-C), 66.26, 66.12 (CH2,2´-C), 60.20, 60.11(C, 8-C), 42.68, 42.42, 41.53, 40.93 (CH2, 4-C, 9-C), 27.07, 25.75, 25.71, 25.55, 25.50, 25.37, 24.43, 24.36 (CH3, Me).
(1´S, 5R/S) 7-(1,1-Dimethylethoxycarbonyl)-8,8-dimethyl-3-(1´,2´-di-O-isopropylidene-1´,2´-dihydroxyethyl)-6-oxo-1-oxa-2,7-diazaspiro[4,4]non-2-ene (5d + 6d)
1H-NMR (CDCl3, 300 MHz): 4.7-4.9 (1H, m), 4.0-4.3 (2H, m), 3.0-3.6 (2H, m), 2.0-2.4 (2H, m), 1.4, 1.5, 1.6 (21H, s, Me). 13C-NMR (CDCl3, 300 MHz): 177.40, 170.50, 170.34, 157.09, 156.82 (C, 3-C, 6-C), 109.74, 109.66 (C, OCO), 85.77, 85.66 (C, 5-C), 82.80 (CMe3), 70.17, 70.09 (CH, 1´-C), 66.30, 66.27 (CH2, 2´-C), 59.47, 59.43 (C, 8-C), 46.53, 46.09, 41.61, 40.82 (CH2, 4-C, 9-C), 27.68, 27.33, 26.42, 26.40, 25.58, 25.53, 24.49, 24.40 (CH3, Me).
(1´S, 5R/S) 7-(1-Methylethenyl)-8,8-dimethyl-3-(1´,2´-di-O-isopropylidene-1´,2´-dihydroxyethyl)-6-oxo-1-oxa-2,7-diazaspiro[4,4]non-2-ene (5e + 6e)
1H-NMR (CDCl3, 300 MHz): 4.9-5.2 (1H, m), 4.0-4.2 (1H, m), 3.4-3.7 (1H, m), 3.1-3.3 (2H, m), 2.3-2.5 (1H, m), 1.3, 1.4, 1.5, 1.7 (15H, s, Me). 13C-NMR (CDCl3, 300 MHz): 168.77, 168.60, 156.56, 156.37 (C, 4-C, 9-C), 138.58, 138.53 (C, C=CH2), 114.24, 113.42, 109.93, 109.15 (C, CH2, OCO, C=CH2), 85.82, 85.74 (C, 5-C), 69.91, 69.89 (CH, 1´-C), 65.93, 65.85 (CH2, 2´-C), 52.87 (C, 8-C), 47.44, 47.02, 40.97, 40.29 (CH2, 4-C, 9-C), 30.34, 27.37, 25.17, 27.12, 25.26, 25.21, 24.89, 24.19, 24.12, 20.73 (CH3, Me).
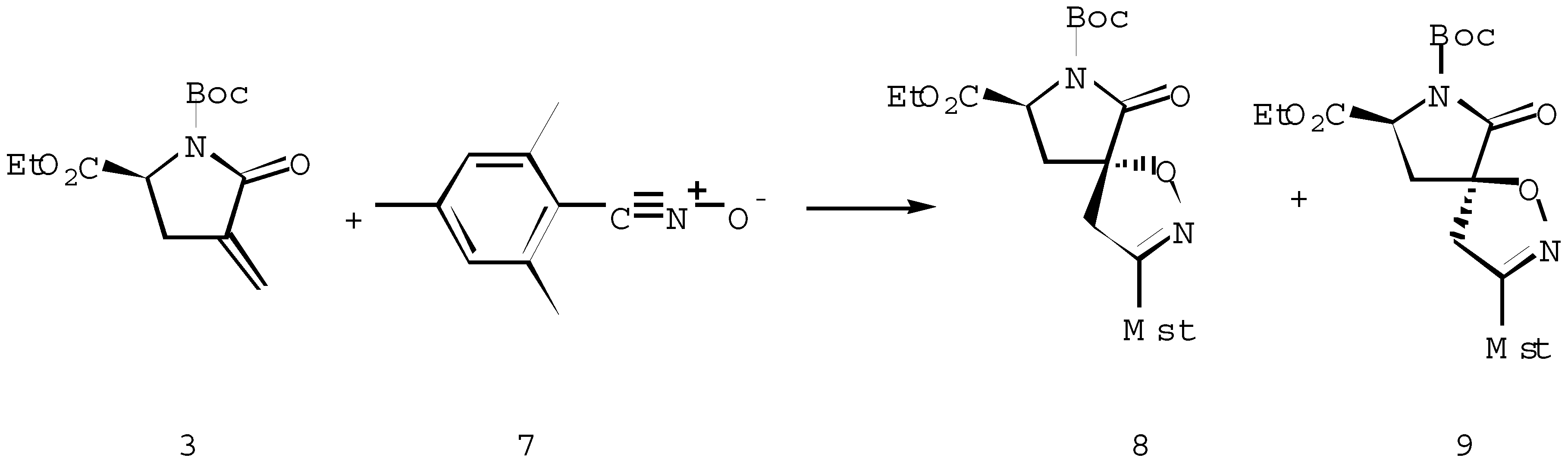
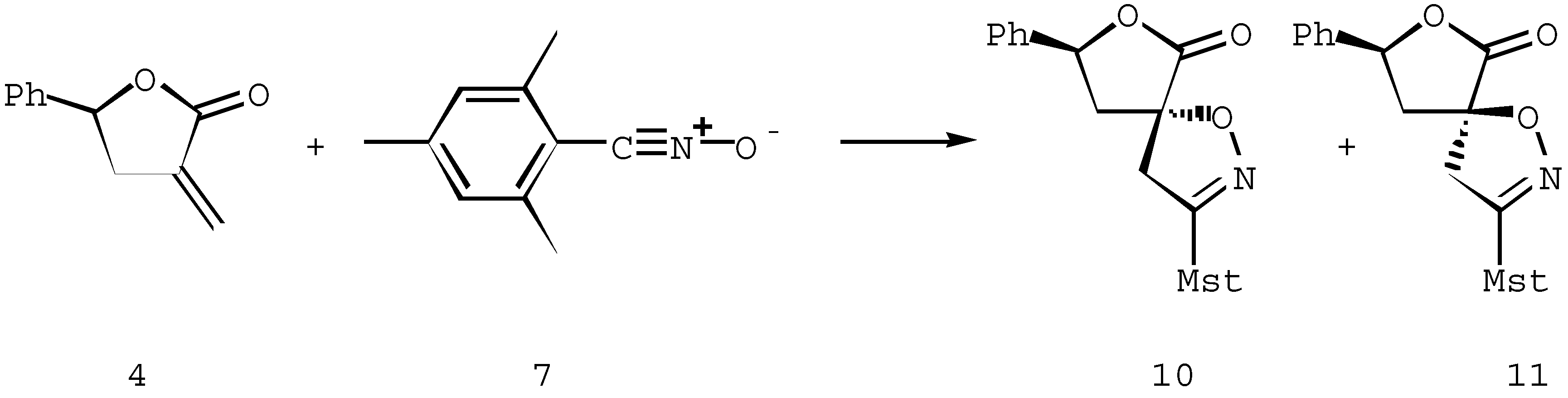
Spiroisoxazolines 8-11. General procedure
The dipolarophile 3 or 4 (1.43 mmol) and mesityl nitrile oxide 7 (1.43 mmol) were dissolved in 5 ml of benzene and warmed (70°C) with stirring until the reaction was complete (TLC). After evaporation of the solvent, pure diastereoisomers were obtained by crystallization or silica gel column chromatography, as described below.
(8S,5S)-7-(1,1-Dimethylethoxycarbonyl)-8-ethoxycarbonyl-3-(2,4,6-trimethylphenyl)-6-oxo-1-oxa-2,7- diazaspiro[4,4]non-2-ene (8)
Yield: 88%. Reaction time, 26h. Pure diastereoisomer 8 was obtained by silica gel chromatography (i-hexane/ethyl acetate 6:1).
1H-NMR (CDCl3, 300 MHz): 6.8 (2H, s, Har), 4.6 (1H, dd, J = 8.4, 5.7 Hz, 8-H), 4.2 (2H, q, J = 7.1 Hz, CH2CH3), 3.8 (1H, d, J = 17.8 Hz, 4-HA), 2.9(1H, d, J = 17.8 Hz, 4- HB), 2.8 (1H, dd, J = 13.9, 8.4 Hz, 9-HA), 2.2 (3H, s, Ph-pMe), 2.2 (6H, s, 2xPh-oMe), 2.1 (1H, dd, J = 13.9, 5.7 Hz, 9-HB), 1.5 (9H, s, CMe3), 1.2 (3H, t, J = 7.1 Hz, CH2Me). 13C-NMR (CDCl3, 300 MHz): 14.1 (CH3, CH2Me), 19.7 (CH3, 2xPh-oMe), 21.1 (CH3, Ph-pMe), 27.9 (CH3, CMe3), 35.1 (CH2, 9-C), 47.0 (CH2, 4-C), 55.8 (CH, 8-C), 62,0 (CH2, CH2Me), 84.6 (C, CMe3), 86.0 (C, 5-C), 124,7 (C, Car), 128.5 (CH, Car), 136.8, 139.2 (C, Car), 149.0 (C, N-CO2), 157.0 (C, 3-C), 170.0 (C, 6-C), 170.8 (C, C-CO2).
Some relevant signals corresponding to a minor isomer (8S,5R)-7- (1,1-dimethylethoxycarbonyl)-8-ethoxycarbon- yl 3-(2,4,6-trimethylphenyl)-6-oxo-1-oxa-2,7-diazaspiro-[4,4]non-2-ene 9 were also clearly observed in the crude original mixture, and characterized by 13C-NMR (CDCl3, 300 MHz): 46.0 (CH2, 4-C), 56.1 (CH, 8-C), 84.1 (C, CMe3), 128.7 (C, Car), 149.2 (C, N-CO2), 169.8 (C, 6-C), 170.0 (C, C-CO2).
3-(2,4,6-Trimethylphenyl)-8-phenyl-6-oxo-1,7-dioxa-2-azaspiro[4,4]non-2-ene (10)
Yield: 90%. Reaction time, 3,5 h. Pure diastereoisomer 10 was obtained by crystallization from hexane/ethanol 2:1. 1H-NMR (CDCl3, 300 MHz): 7.3-7.4 (5H, m, Har), 6.9 (2H, m, Har), 5.7 (1H, dd, J = 9.7 , 5.5 Hz, 8-H), 3.8 (1H, d, J = 17.6 Hz, 4-HA), 3.1 (1H, d, J= 17.6 Hz, 4-HB), 3.1 (1H, dd, J = 13.8, 5.5 Hz, 9-HA), 2.3 (1H, dd, J = 13.8, 9.7 Hz, 9-HB), 2.3 (s, 3H, Me), 2.3 (s, 6H, 2xMe). 13C-NMR (CDCl3, 300 MHz): 177.3 (6-C), 157.4 (3-C), 139.2, 137.7, 136.7, 128.8, 128.4, 125.8, 125.4, 124.6 (Car), 85.4, 79.3 (5-C, 8-C), 45.3, 43.6 (4-C, 9-C), 21.0, 19.6 (Me).
Some relevant signals corresponding to a minor isomer 11 were also clearly observed in the crude original mixture. 1H-NMR (CDCl3,300 MHz): 3.7 (1H, d, J = 17.4 Hz, 4-HA), 3.3 (1H, d, J = 17.4 Hz, 4-HB). 13C-NMR (CDCl3, 300 MHz): 84.6, 77.9 (5-C, 8-C), 47.8, 42.9 (4-C, 9-C).