General Details
Chemicals and reagent were purchased from Fluka Chemie Co. and used without further purification. The ethyl 2–aminothiophene–3–carboxylates were prepared by Gewald's method [
12] . Melting points of prepared compounds
1–
6,
7–
12 were measured on a Boetius Rapido PHMK 79/2106 (Wägetechnik) instrument and are presented in the
Table 4. The purity of compounds was controlled by CHN elemental analysis on an instrument 1102 (Erba), by determinations of selenium on spectrometer ICP AES 7500 (Unicam) and the found values correspond to the calculated ones. TLC was carried out on Silufol UV 254 plates (Kavalier, Votice) and the detection with Fluotes Universal (Qurtzlampen, Hanau) and with iodine vapors. Chloroform and diethylether in a container saturated with vapors of the used solvent was used as an eluent.
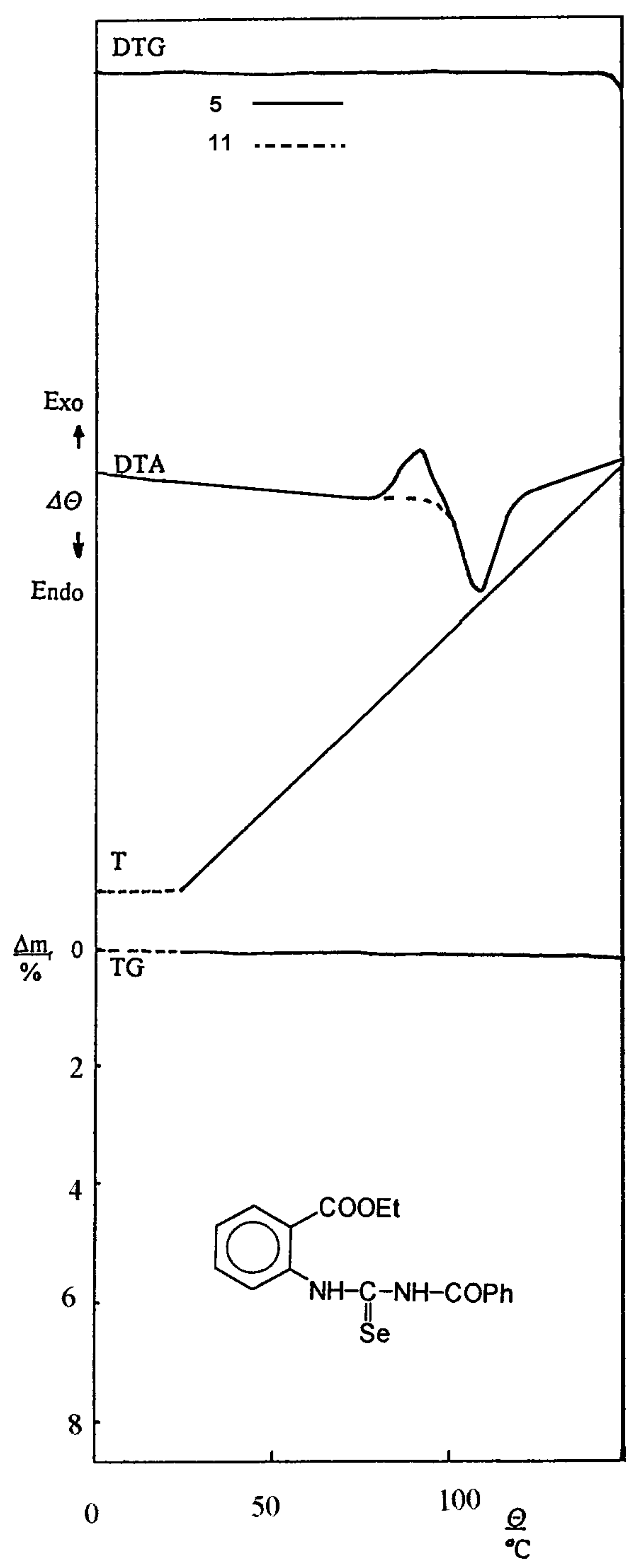
The thermal behaviour of compounds was followed with Derivatograph OD–102 (MOM, Budapest). The analyses were provided in about 100 mg samples in a platinum crucible without a lid in a stationary atmosphere of the furnace, as a standard material preglowed α–Al2O3 was used. The measurements were carried out at 150 °C, TG 100 mg, DTG 1/10 and DTA 1/5. The heating rate was 6 °C min-1.
FTIR spectra were taken on a spectrometer Genesis (UNICAm) in potassium bromide pellets.
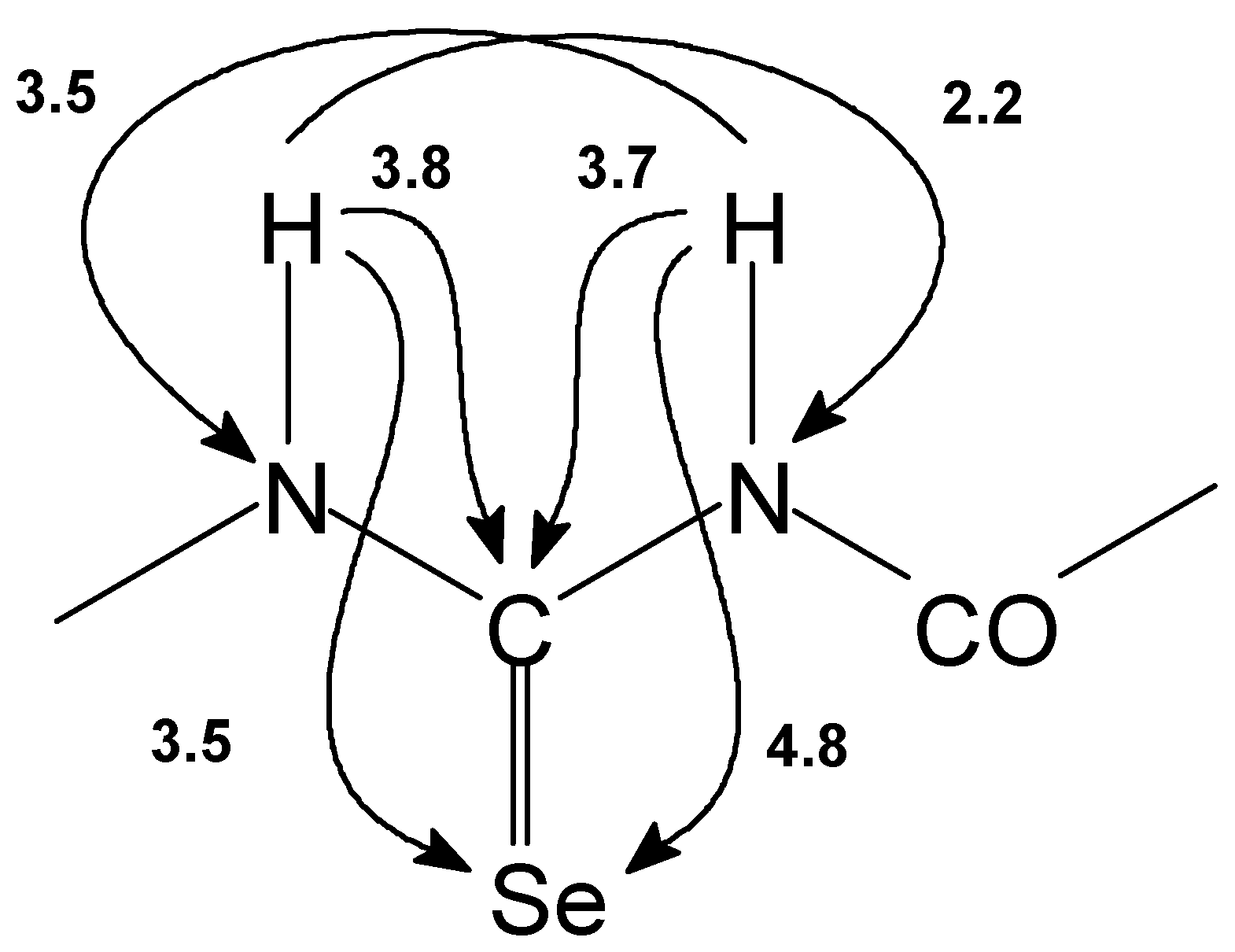
NMR spectra were measured on a Bruker Avance DRX–500 spectrometer. The 13C and 1H spectra were referenced to tetramethylsilane used as an internal standard or to the solvent signals of CDCl3 and of residual CHCl3 at 77.00 ppm (13C) and 7.27 ppm (1H), respectively. 77Se chemical shifts were referenced to H2SeO3 (1282 ppm) and SeOCl2 (1479 ppm) and 15N chemical shifts to liquid ammonia (0 pppm) used as external standards. Spectral width : 9000 Hz for 1H, 27500 Hz for 13C and 38000 Hz for 77Se.
HETCOR and COLOC spectra: Bruker standard sequences; relaxation delay 2.5 s; delay for evolution of direct 3.45 ms and long range 40 ms
1H–
13C couplings; WALTZ16 decoupling during acquisition, spectral widths were taken from the corresponding 1D spectra; data
table 2 k x 0.5 k.
1H–
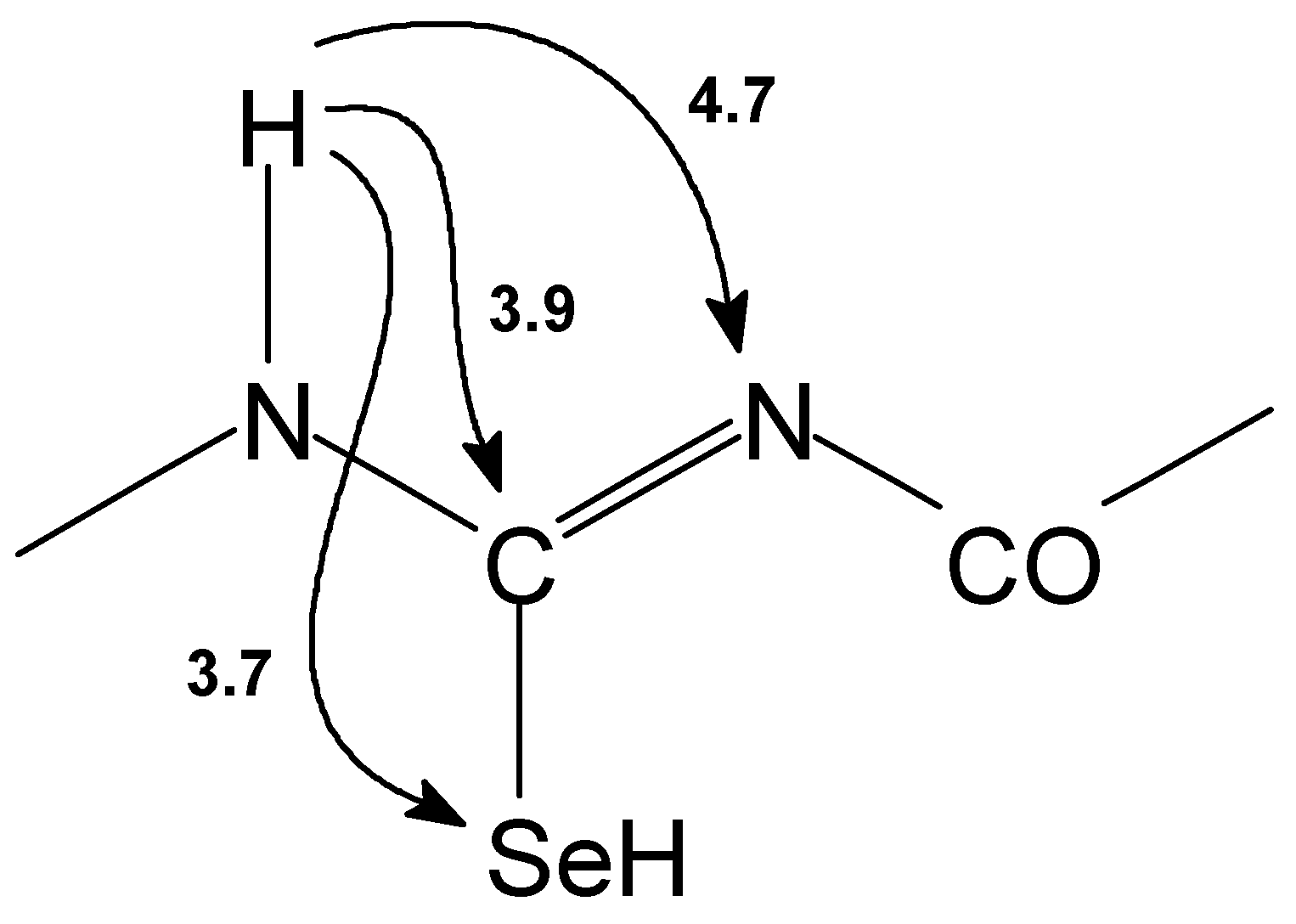
15N HMBC spectra [
10]: relaxation delay 2.5 s; delay for evolution of long range couplings 100 ms, postgradient recovery 100 μs, gradient pulses 1 ms, gradient ratios 42:18:30 G/cm;
1H–
77Se GHMBC [
10];
1H–
15N and
1H–
77Se GSQMBC [
10,
11].
UV–VIS spectra were taken on a spectrophotometer SP 1800 (Unicam) in chloroform solutions.
Irradiation of reaction mixtures was performed with a filament lamp 60 W (Tesla).
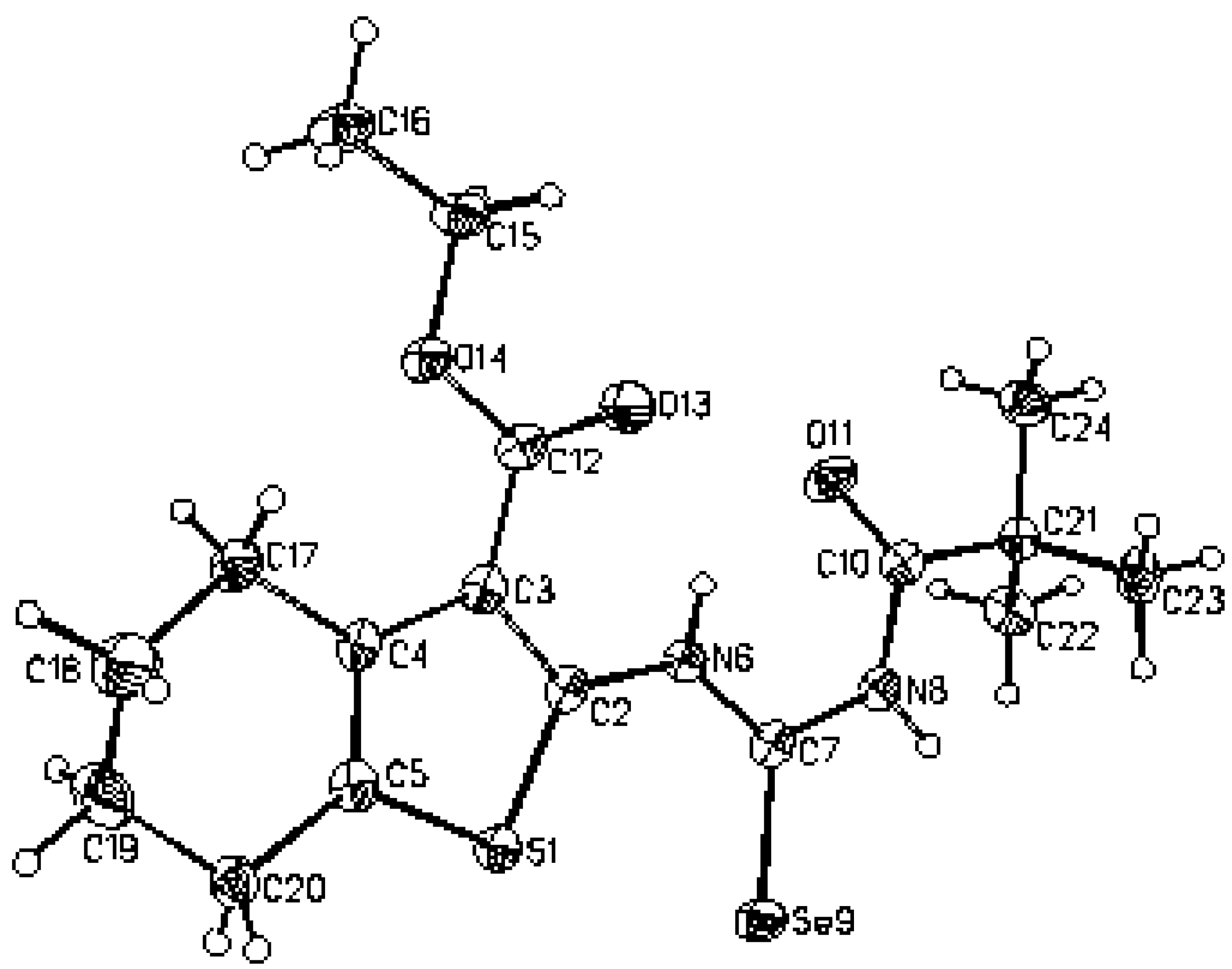
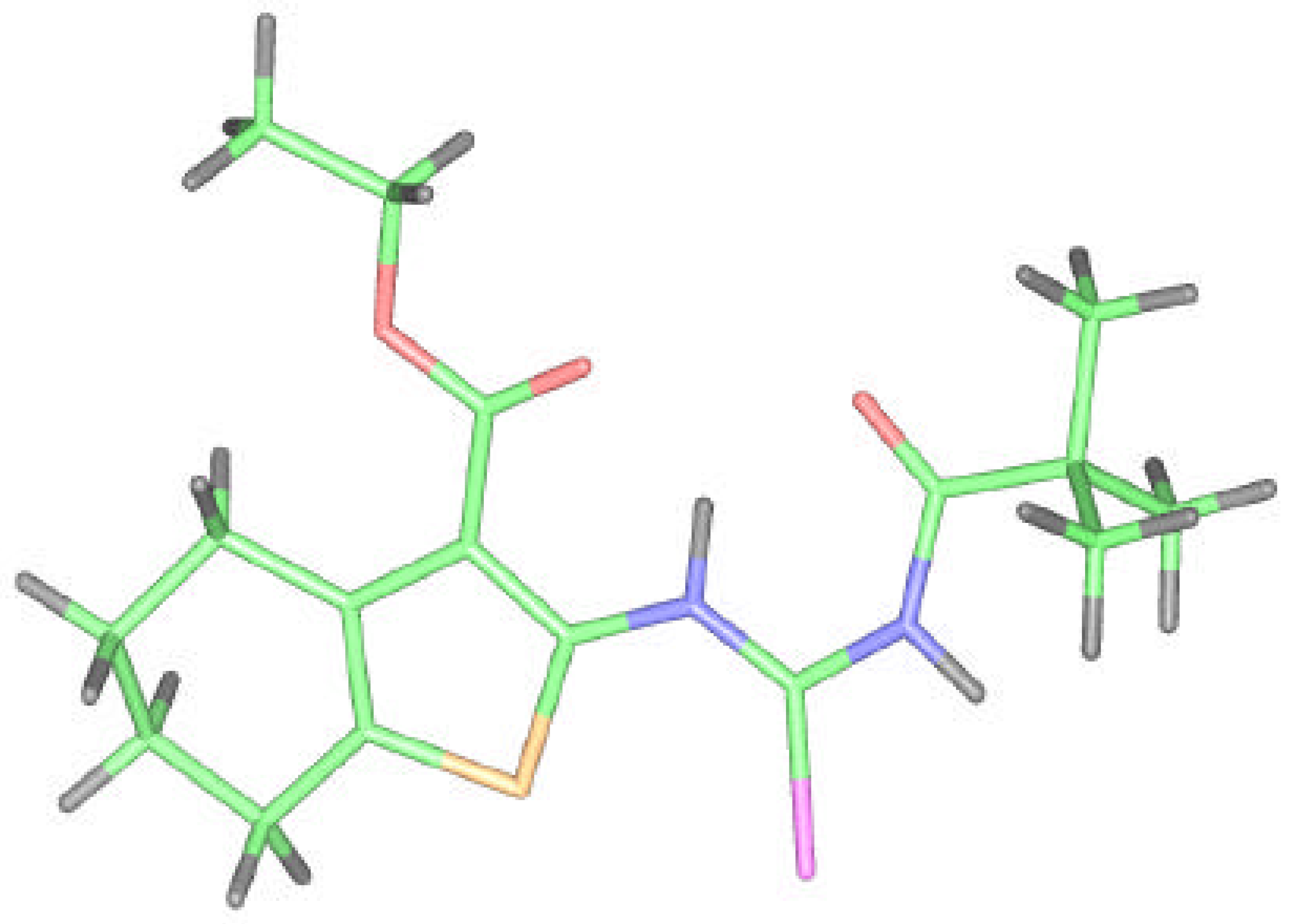
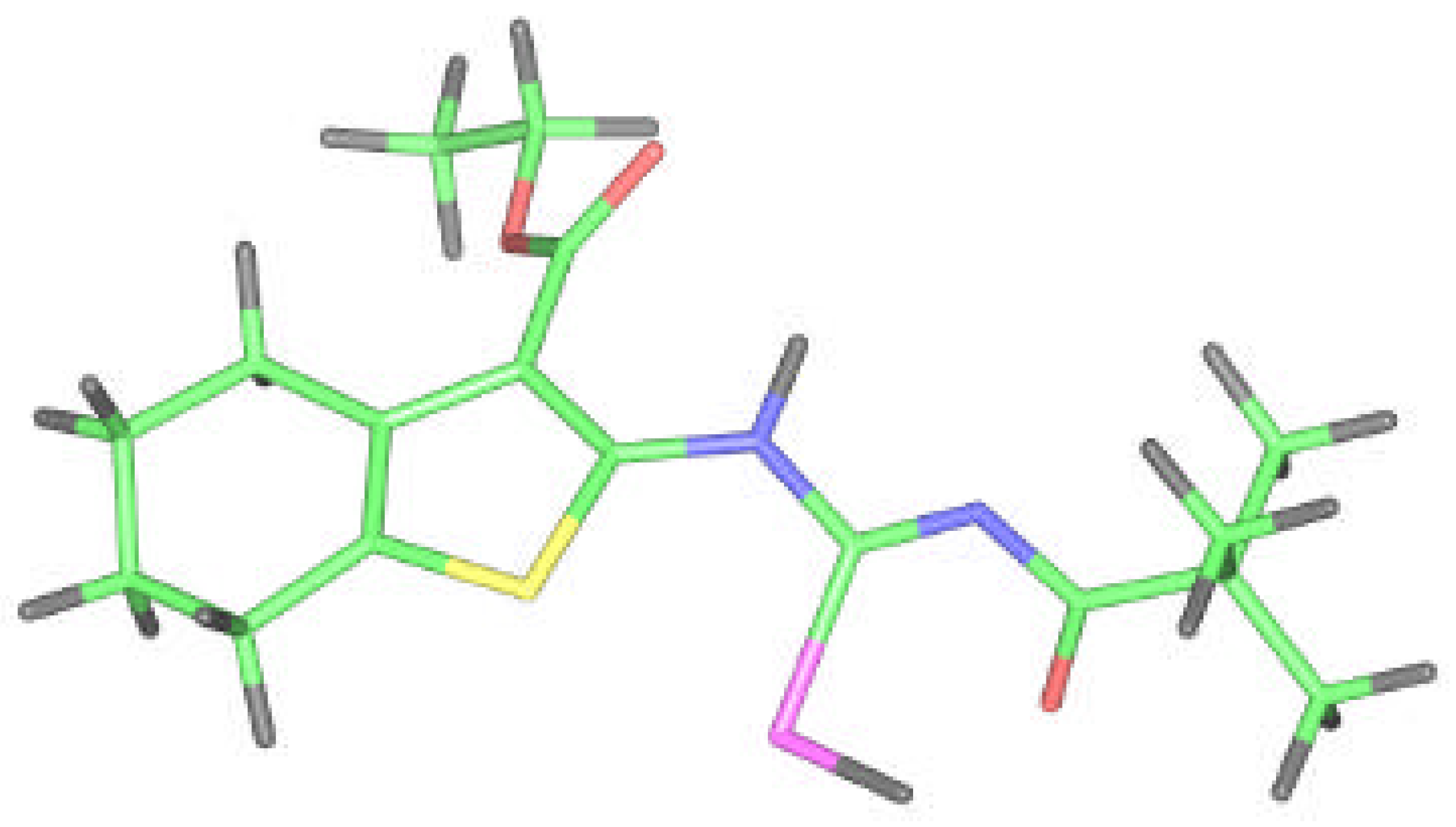
X–Ray structural data of compound
2 were collected with a KUMA KM–4 kappa four–circles diffractometer. The structure was solved by direct methods using SHELXS–86 [
13] and refined on
F2 for all reflections using SHELXL–93 [
14]. Crystal suitable for X–ray was obtained by recrystallization from chloroform in the form of yellow monoclinic needles.
Geometry optimization of structures 2, 8 was performed at ab initio level of quantum chemical calculation, RHF/DZVP and DFT/VWN/DZVP, respectively.
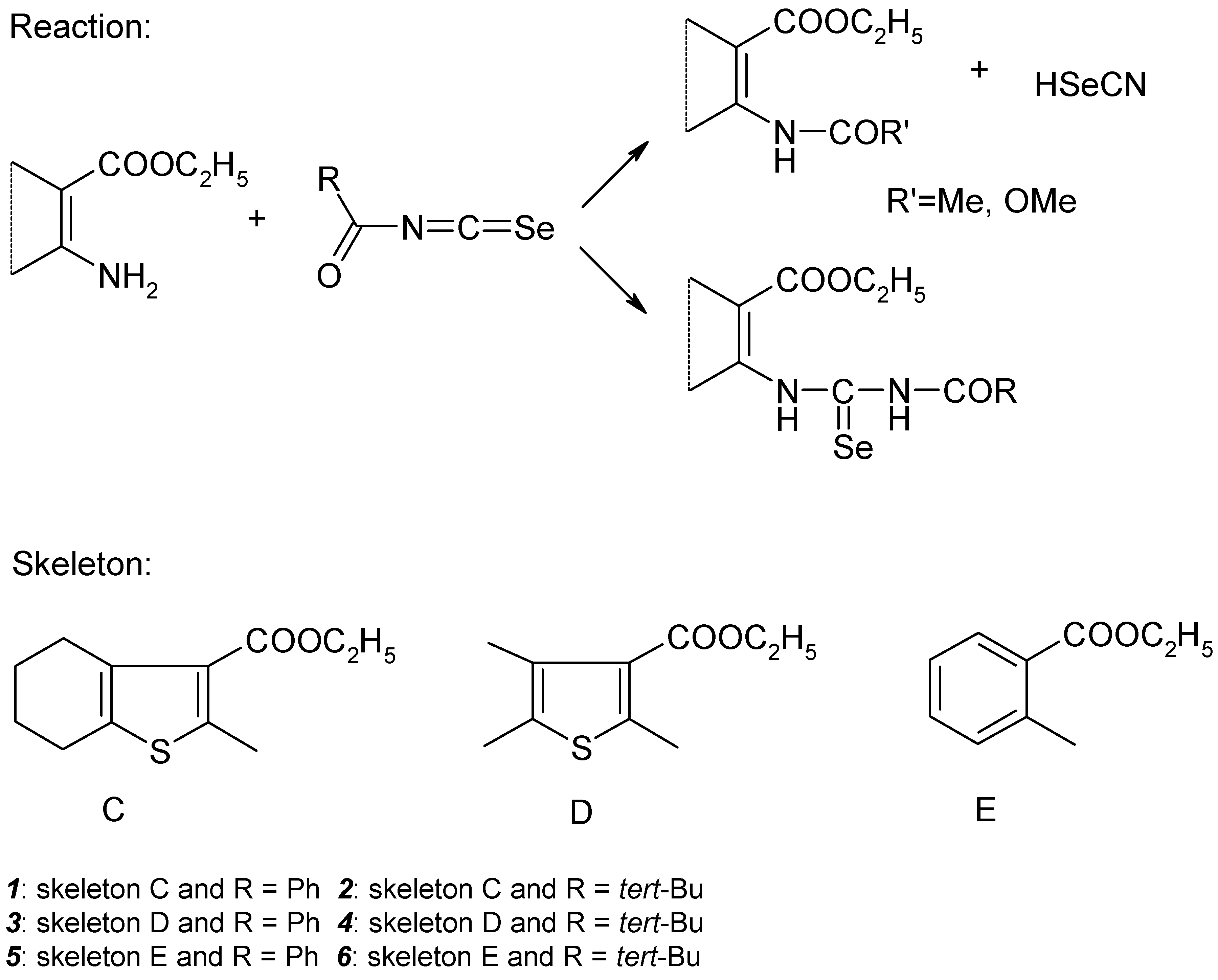
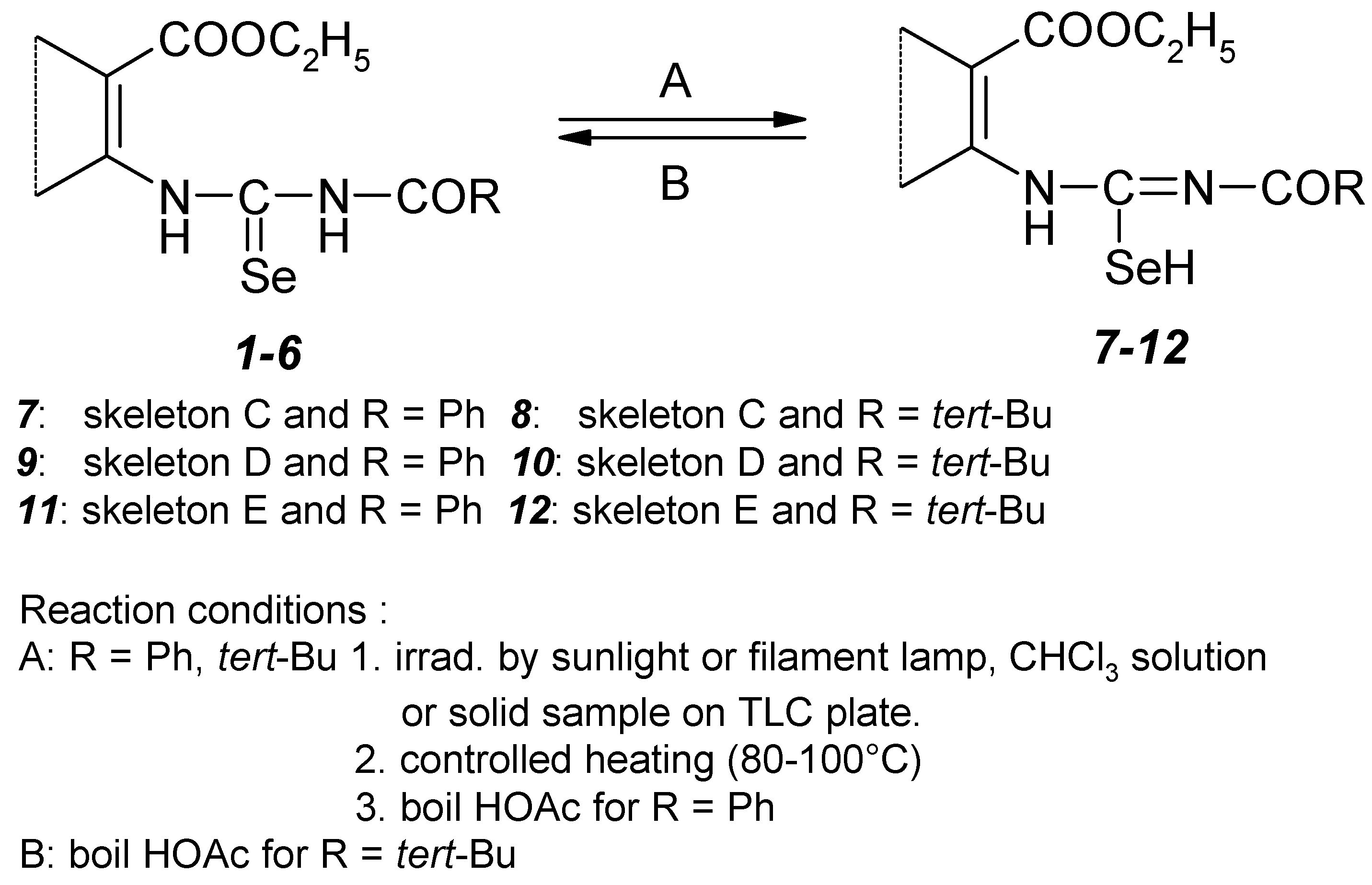
Acylselenoureas 1–6
Addition of aminoester to acylisoselenocyanate (a)
Potassium selenocyanate (7.63 g, 53 mmol) was dissolved in acetone (50 ml, dried 24 h with anhydrous calcium chloride and distilled) under stirring at room temperature. Benzoylchloride (7.45 g, 53 mmol) or pivaloylchloride (6.39 g, 53 mmol) dissolved in acetone (20 ml) was dropwise added. The reaction mixture was stirred under inert gas (argon, nitrogen) for 10 min, the precipitated potassium chloride was filtered off by suction and washed with acetone (10 ml). The corresponding aminoester (50 mmol) was poured by benzoyl– or pivaloylisoselenocyanate in acetone solution connected acetone part and the formed solution reacted at room temperature. After 15–30 min (TLC control) the reaction mixture was dried on an evaporator and the crude product dissolved in chloroform at a temperature of about 4 °C. The formed suspension was filtered with charcoal, the filtrate separated from colloid selenium and concentrated to 1/5 original volume and mixed with an equivalent of petroleum ether. The precipitated crystals were filtered off by suction, washed with petroleum ether and finally with cold methanol. The product was dried in vacuo.
Acid catalyzed retroisomerization of pivaloylisoselenoureas 8, 10, 12 (b)
Pivaloylisoselenoureas 8, 10, 12 (5 mmol) dissolved in acetic acid (50 ml) were stirred for 5–10 min (TLC monitoring). The pure product was obtained after removing of acetic acid on an evaporator.
Ethyl 2–(3–benzoylselenoureido)–4,5,6,7–tetrahydrobenzo[b]thiophene–3–carboxylate (1)
M.p. 172–174 °C; Yield 21.9 g (95%); FTIR 3410, 3330 (NH), 1680, 1250 (COOC), 1670, 1560 (NHCO, amide I, II), 1532, 965 (NHCSe, selenoamide III, I) cm-1; 1H NMR (CDCl3) 1.40 (3H, t, J 7.1 Hz, OCH2CH3), 1.78–1.81 (4H, m, 5–CH2 and 6–CH2), 2.65 (2H, t, J 5.0 Hz, 4–CH2), 2.84 (2H, t, J 6.0 Hz, 7–CH2), 4.46 (2H, q, J 7.1 Hz, OCH2CH3), 7.50–7.94 (5H, m, C6H5), 9.48 (1H, s, H(N–8)), 15.12 (1H, s, H(N–6)); 13C NMR (CDCl3) 14.41 (CH3, OCH2CH3), 22.81 (C–5 and C–6), 24.57 (C–4), 26.38 (C–7), 60.89 (CH2, OCH2CH3), 117.70 (C–3), 127.74 (C–2' and C–6', C6H5), 128.37 (C–4, thiophene), 129.04 (C–3' and C–5', C6H5), 131.36 (C–1', C6H5), 132.42 (C–5, thiophene), 133.57 (C–4', C6H5), 146.84 (C–2), 164.75 (C=O, COC6H5), 165.20 (C=O, COOCH2CH3), 174.47 (C=Se, 1JC,Se 223 Hz); 15N NMR (CDCl3) 156.20 (N–6), 164.80 (N–8); 77Se NMR (CDCl3) 480; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 266/4.23, 286/4.16, 386/4.08.
Ethyl 2–(3–pivaloylselenoureido)–4,5,6,7–tetrahydrobenzo[b]thiophene–3–carboxylate (2)
M.p. 153–155 °C; Yield (a) 20.5 g (93%), (b) 21.8 (99%); FTIR 3280, 3200 (NH), 1685, 1235 (COOC), 1660, 1555 (NHCO, amide I, II), 1525, 980 (NHCSe, selenoamide III, I) cm-1; 1H NMR (CDCl3) 1.33 (9H, s, CMe3), 1.38 (3H, t, J 7.1 Hz, OCH2CH3), 1.78–1.81 (4H, m, 5–CH2 and 6–CH2), 2.65 (2H, t, J 5.0 Hz, 4–CH2), 2.83 (2H, t, J 6.0 Hz, 7–CH2), 4.44 (2H, q, J 7.1 Hz, OCH2CH3), 8.89 (1H, s, H(N–8)), 14.93 (1H, s, H(N–6)); 13C NMR (CDCl3) 14.33 (CH3, OCH2CH3), 22.79 (C–5 and C–6), 24.54 (C–4), 26.36 (C–7), 26.98 (CH3, C(CH3)3, 39.75 (C(CH3)3, 60.82 (CH2, OCH2CH3), 117.74 (C–3), 128.42 (C–4, thiophene), 132.40 (C–5, thiophene), 146.76 (C–2, thiophene), 165.09 (C=O, COOCH2CH3), 176.98 (C=O, COCMe3), 174.83 (C=Se, 1JC,Se 220 Hz); 15N NMR (CDCl3) 156.1 (N–6), 163.9 (N–8); 77Se NMR (CDCl3) 459; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 250/4.70, 274/4.66, 370/4.99.
Ethyl 2–(3–benzoylselenoureido)–4,5–dimethylthiophene–3–carboxylate (3)
M.p. 173–175 °C; Yield 19.9 g (92%); FTIR 3220 (NH), 1685, 1245 (COOC), 1675, 1557 (NHCO, amide I, II), 1522, 985 (NHCSe, selenoamide III, I) cm-1; 1H NMR (CDCl3) 1.42 (3H, t, J 7.1 Hz, OCH2CH3), 2.29 (3H, s, CH3, C–4 thiophene), 2.32 (3H, s, CH3, C–5 thiophene), 4.50 (2H, q, J 7.1 Hz, OCH2CH3), 7.53–7.95 (5H, m, C6H5), 9.42 (1H, s, H(N–8)), 15.11 (1H, s, H(N–6)); 13C NMR (CDCl3) 12.70 (CH3, C–4 thiophene), 14.39 (CH3, OCH2CH3), 14.40 (CH3, C–5 thiophene), 61.06 (CH2, OCH2CH3), 119.02 (C–3), 125.22 (C–4), 127.74 (C–2' and C–6', C6H5), 129.16 (C–3' and C–5', C6H5), 130.77 (C–5), 131.45 (C–1', C6H5), 133.68 (C–4', C6H5), 145.82 (C–2), 164.83 (C=O, COC6H5), 165.26 (C=O, COOCH2CH3), 174.45 (C=Se, 1JC,Se 222 Hz); 77Se NMR (CDCl3) 474; UV–VIS (CHCl3, λmax, nm/log ε, log(m2 mol-1)) 270/4.21, 306/4.14, 386/4.06.
Ethyl 2–(3–pivaloylselenoureido)–4,5–dimethylthiophene–3–carboxylate (4)
M.p. 183–186 °C; Yield (a) 18.4 g (89%), (b) 20.3 (98%); FTIR 3348, 3250, 3182 (NH), 1688, 1239 (COOC), 1660, 1563 (NHCO, amide I, II), 1515, 942 (NHCSe, selenoamide III, I) cm-1; 1H NMR (CDCl3) 1.33 (9H, s, CMe3), 1.39 (3H, t, J 7.1 Hz, OCH2CH3), 2.27 (3H, s, CH3, C–4 thiophene), 2.29 (3H, s, CH3, C–5 thiophene), 4.46 (2H, q, J 7.1 Hz, OCH2CH3), 8.89 (1H, s, H(N–8)), 14.92 (1H, s, H(N–6)); 13C NMR (CDCl3) 12.67 (C–4), 14.32 (CH3, OCH2CH3), 14.41 (C–5), 27.00 (CH3, C(CH3)3, 39.77 (C(CH3)3, 60.97 (CH2, OCH2CH3), 118.87 (C–3), 125.10 (C–4), 130.64 (C–5, thiophene), 145.75 (C–2, thiophene), 165.11 (C=O, COOCH2CH3), 177.07 (C=O, COCMe3), 174.69 (C=Se); 77Se NMR (CDCl3) 458; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 250/3.98, 318/3.89, 382/3.68.
Ethyl 2–(3–benzoylselenoureido)benzoate (5)
M.p. 107–110 °C; Yield 16.9 g (85 %); FTIR 3380, 3220 (NH), 1700, 1265 (COOC), 1670, 1540 (NHCO, amide I, II), 1520, 980 (NHCSe, selenoamide III, I) cm-1; 1H NMR (CDCl3) 1.38 (3H, t, J 7.1 Hz, OCH2CH3), 4.40 (2H, q, J 7.1 Hz, OCH2CH3), 7.37–8.45 (9H, m, C6H5 and C6H4), 9.61 (1H, s, H(N–8)), 13.70 (1H, s, H(N–6)); 13C NMR (CDCl3) 14.22 (CH3, OCH2CH3), 61.59 (CH2, OCH2CH3), 123.49 (C–1), 126.87 (C–5), 127.18 (C–3), 127.77 (C–2' and C–6', C6H5), 129.08 (C–3' and C–5', C6H5), 130.86 (C–6), 131.36 (C–1', C6H5), 133.71 (C–4', C6H5), 139.27 (C–2), 165.75 (C=O, COOCH2CH3), 165.83 (C=O, COC6H5), 180.22 (C=Se, 1JC,Se 225 Hz); 77Se NMR (CDCl3) 383; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 247/4.41, 310/4.92, 362/4.70.
Ethyl 2–(3–pivaloylselenoureido)benzoate (6)
M.p. 109–112 °C; Yield (a) 15.4 g (82%), (b) 18.4 (98%); FTIR 3256, 3195 (NH), 1715, 1256 (COOC), 1680, 1546 (NHCO, amide I, II), 1520, 979 (NHCSe, selenoamide III, I) cm-1;1H NMR (CDCl3) 1.33 (9H, s, CMe3), 1.37 (3H, t, J 7.1 Hz, OCH2CH3), 4.39 (2H, q, J 7.1 Hz, OCH2CH3), 7.35–8.37 (4H, m, C6H4), 8.93 (1H, s, H(N–8)), 13.49 (1H, s, H(N–6)); 13C NMR (CDCl3) 14.09 (CH3, OCH2CH3), 26.84 (CH3, C(CH3)3, 39.80 (C(CH3)3, 61.43 (CH2, OCH2CH3), 123.56 (C–1), 126.76 (C–5), 127.10 (C–3), 130.76 (C–6), 132.17 (C–4), 139.07 (C–2), 165.55 (C=O, COOCH2CH3), 178.04 (C=O, COCMe3), 180.32 (C=Se); 77Se NMR (CDCl3) 384; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 276/4.73, 316/4.95, 379/4.82.
| Crystal data and structure refinement of
2 |
| Empirical formula | C17 H24 N2 O3 S Se |
| Formula weight | 415.4 |
| Temperature | 153(2) K |
| Wave length | 0.71073 Å |
| Crystal system | monoclinic |
| Space group | P 2(1)/c |
| Unit cell dimensions | a = 9.198(2) Å
b = 10.006(2) Å
c = 19.864(4) Å | alpha = 90 deg.
beta = 99.98(3) deg.
gamma = 90 deg. |
| Volume | 1800.5(6) Å3 |
| Z | 4 |
| Density (calculated) | 1.532 mg/m3 |
| Absorption coefficient | 2.219 mm-1 |
| F(000) | 856 |
| Crystallize | 0.80 x 0.80 x 0.40 mm |
| Theta range for data collection | 2.08 to 25.07 deg. |
| Index ranges | 0<=h<=10, 0<=k<=11, -23<=l<=23 |
| Reflections collected | 3386 |
| Independent reflections | 3176 [R(int) = 0.0555] |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 3176 / 0 / 303 |
| Goodness-of-fit on F2 | 0.991 |
| Final R indices [I> sigma(I)] | R1 = 0.0443, wR2 = 0.1134 |
| R indices (all data) | R1 = 0.0574, wR2 = 0.1231 |
| Largest diff. peak and hole | 1.467 and -0.902 eÅ-3 |
Table 5.
Atomic coordinates (x 104) and equivalent isotropic displacement parameters (Å2 x 103). U(eq) is defined as one third of the trace of the orthogonalized Uij tensor.
Table 5.
Atomic coordinates (x 104) and equivalent isotropic displacement parameters (Å2 x 103). U(eq) is defined as one third of the trace of the orthogonalized Uij tensor.
| Atom | x | y | z | U(eq) |
| S(1) | 1950(1) | 4842(1) | 576(1) | 19(1) |
| C(2) | 2349(4) | 4526(4) | -225(2) | 16(1) |
| C(3) | 3193(4) | 5540(4) | -441(2) | 18(1) |
| C(4) | 3518(4) | 6581(4) | 61(2) | 18(1) |
| C(5) | 2910(4) | 6330(4) | 622(2) | 19(1) |
| N(6) | 1895(3) | 3397(3) | -617(2) | 17(1) |
| C(7) | 1200(4) | 2309(4) | -444(2) | 17(1) |
| N(8) | 934(4) | 1293(3) | -932(2) | 19(1) |
| Se(9) | 566(1) | 2040(1) | 360(1) | 26(1) |
| C(10) | 1154(4) | 1308(4) | -1602(2) | 18(1) |
| O(11) | 1691(4) | 2268(3) | -1839(2) | 31(1) |
| C(12) | 3664(4) | 5480(4) | -1106(2) | 19(1) |
| O(13) | 3418(3) | 4544(3) | -1504(2) | 29(1) |
| O(14) | 4407(3) | 6563(3) | -1246(1) | 20(1) |
| C(15) | 4908(5) | 6558(5) | -1900(2) | 24(1) |
| C(16) | 5780(5) | 7806(5) | -1941(3) | 30(1) |
| C(17) | 4390(5) | 7842(4) | 5(2) | 25(1) |
| C(20) | 2996(5) | 7220(4) | 1236(2) | 24(1) |
| C(21) | 664(4) | 59(4) | -2019(2) | 18(1) |
| C(22) | -986(4) | -192(5) | -2023(2) | 24(1) |
| C(23) | 1588(5) | -1129(5) | -1715(3) | 28(1) |
| C(24) | 901(5) | 278(5) | -2753(2) | 29(1) |
| C(18) | 4728(10) | 8562(7) | 684(4) | 78(3) |
| C(19) | 3602(10) | 8565(7) | 1081(4) | 69(2) |
Table 6.
Bond lengths [Å] and angles [deg.].
Table 6.
Bond lengths [Å] and angles [deg.].
| S(1)-C(2) | 1.722(4) | C(2)-S(1)-C(5) | 91.1(2) |
| S(1)-C(5) | 1.725(4) | C(3)-C(2)-N(6) | 123.3(4) |
| C(2)-C(3) | 1.389(5) | C(3)-C(2)-S(1) | 111.8(3) |
| C(2)-N(6) | 1.394(5) | N(6)-C(2)-S(1) | 124.9(3) |
| C(3)-C(4) | 1.436(5) | C(2)-C(3)-C(4) | 112.1(3) |
| C(3)-C(12) | 1.461(6) | C(2)-C(3)-C(12) | 121.2(4) |
| C(4)-C(5) | 1.354(6) | C(4)-C(3)-C(12) | 126.8(4) |
| C(4)-C(17) | 1.510(5) | C(5)-C(4)-C(3) | 111.7(3) |
| C(5)-C(20) | 1.502(6) | C(5)-C(4)-C(17) | 121.1(4) |
| N(6)-C(7) | 1.338(5) | C(3)-C(4)-C(17) | 127.2(4) |
| C(7)-N(8) | 1.398(5) | C(4)-C(5)-C(20) | 126.0(4) |
| C(7)-Se(9) | 1.812(4) | C(4)-C(5)-S(1) | 113.3(3) |
| N(8)-C(10) | 1.380(5) | C(20)-C(5)-S(1) | 120.7(3) |
| C(10)-O(11) | 1.212(5) | C(7)-N(6)-C(2) | 128.8(4) |
| C(10)-C(21) | 1.523(5) | N(6)-C(7)-N(8) | 116.6(3) |
| C(12)-O(13) | 1.222(5) | N(6)-C(7)-Se(9) | 126.1(3) |
| C(12)-O(14) | 1.336(5) | N(8)-C(7)-Se(9) | 117.4(3) |
| O(14)-C(15) | 1.452(5) | C(10)-N(8)-C(7) | 128.7(3) |
| C(15)-C(16) | 1.494(6) | O(11)-C(10)-N(8) | 121.4(4) |
| C(17)-C(18) | 1.514(7) | O(11)-C(10)-C(21) | 122.8(4) |
| C(20)-C(19) | 1.508(7) | N(8)-C(10)-C(21) | 115.8(3) |
| C(21)-C(23) | 1.523(6) | O(13)-C(12)-O(14) | 122.0(4) |
| C(21)-C(24) | 1.528(6) | O(13)-C(12)-C(3) | 124.5(4) |
| C(21)-C(22) | 1.538(5) | O(14)-C(12)-C(3) | 113.5(3) |
| C(18)-C(19) | 1.406(9) | C(12)-O(14)-C(15) | 116.0(3) |
| | | O(14)-C(15)-C(16) | 107.6(4) |
| | | C(4)-C(17)-C(18) | 111.2(4) |
| | | C(5)-C(20)-C(19) | 109.5(4) |
| | | C(10)-C(21)-C(23) | 109.5(3) |
| | | C(10)-C(21)-C(24) | 108.8(3) |
| | | C(23)-C(21)-C(24) | 109.1(4) |
| | | C(10)-C(21)-C(22) | 109.6(3) |
| | | C(23)-C(21)-C(22) | 110.9(4) |
| | | C(24)-C(21)-C(22) | 108.8(3) |
| | | C(19)-C(18)-C(17) | 116.3(6) |
| | | C(18)-C(19)-C(20) | 116.5(6) |
Table 7.
Symmetry transformations used to generate equivalent atoms: anisotropy displacement parameters (Å2 x 103). The anisotropic displacement factor exponent takes the form: -2 pi2 [h2 a*2 U11 + ... + 2 h k a* b* U12].
Table 7.
Symmetry transformations used to generate equivalent atoms: anisotropy displacement parameters (Å2 x 103). The anisotropic displacement factor exponent takes the form: -2 pi2 [h2 a*2 U11 + ... + 2 h k a* b* U12].
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
| S(1) | 16(1) | 21(1) | 20(1) | -2(1) | 2(1) | -5(1) |
| C(2) | 9(2) | 19(2) | 19(2) | 1(2) | -4(1) | -1(2) |
| C(3) | 9(2) | 20(2) | 23(2) | 1(2) | -2(1) | -1(2) |
| C(4) | 10(2) | 17(2) | 23(2) | -1(2) | -3(2) | -3(2) |
| C(5) | 10(2) | 24(2) | 22(2) | -2(2) | -3(2) | -3(2) |
| N(6) | 11(2) | 20(2) | 18(2) | -2(1) | -1(1) | -4(1) |
| C(7) | 8(2) | 20(2) | 20(2) | 1(2) | -4(1) | -2(1) |
| N(8) | 16(2) | 18(2) | 22(2) | -2(1) | 1(1) | -6(1) |
| Se(9) | 30(1) | 26(1) | 23(1) | -2(1) | 7(1) | -13(1) |
| C(10) | 9(2) | 23(2) | 20(2) | 1(2) | -3(1) | -1(2) |
| O(11) | 44(2) | 25(2) | 23(2) | -1(1) | 6(1) | -18(1) |
| C(12) | 9(2) | 24(2) | 20(2) | 1(2) | -3(1) | -4(2) |
| O(13) | 31(2) | 30(2) | 25(2) | -6(1) | 5(1) | -14(1) |
| O(14) | 14(1) | 23(2) | 22(1) | -1(1) | 2(1) | -8(1) |
| C(15) | 18(2) | 32(2) | 22(2) | -3(2) | 5(2) | -9(2) |
| C(16) | 19(2) | 39(3) | 32(3) | 2(2) | 6(2) | -11(2) |
| C(17) | 25(2) | 25(2) | 24(2) | -3(2) | 2(2) | -11(2) |
| C(20) | 24(2) | 23(2) | 25(2) | -5(2) | 5(2) | -3(2) |
| C(21) | 9(2) | 20(2) | 23(2) | -1(2) | 1(2) | -2(2) |
| C(22) | 9(2) | 35(3) | 27(2) | -7(2) | -1(2) | -7(2) |
| C(23) | 20(2) | 23(2) | 41(3) | 1(2) | 4(2) | 3(2) |
| C(24) | 31(3) | 32(3) | 26(2) | 6(2) | 7(2) | 6(2) |
| C(18) | 133(7) | 55(4) | 57(4) | -29(3) | 50(4) | -69(5) |
| C(19) | 111(6) | 52(4) | 58(4) | -36(3) | 53(4) | -56(4) |
Table 8.
Hydrogen coordinates (x 104) and isotropic displacement parameters (Å2 x 103).
Table 8.
Hydrogen coordinates (x 104) and isotropic displacement parameters (Å2 x 103).
| Atom | x | y | z | U(eq) |
| H(6A) | 2101(53) | 3386(50) | -997(27) | 26(13) |
| H(8A) | 502(51) | 632(50) | -784(23) | 21(12) |
| H(15B) | 5501(58) | 5735(56) | -1927(26) | 37(14) |
| H(15A) | 4059(60) | 6476(54) | -2267(28) | 37(14) |
| H(16C) | 5182(64) | 8588(61) | -1873(28) | 45(16) |
| H(16B) | 6492(69) | 7793(59) | -1569(32) | 48(17) |
| H(16A) | 6190(53) | 7805(48) | -2354(26) | 27(12) |
| H(17B) | 3836(59) | 8512(57) | -338(28) | 41(15) |
| H(17A) | 5437(62) | 7591(57) | -97(27) | 40(14) |
| H(20B) | 3504(60) | 6831(53) | 1620(29) | 36(14) |
| H(20A) | 2063(65) | 7298(58) | 1361(28) | 44(15) |
| H(22C) | -1573(61) | 561(60) | -2217(28) | 43(15) |
| H(22B) | -1236(67) | -872(67) | -2327(33) | 58(19) |
| H(22A) | -1170(52) | -347(49) | -1582(26) | 27(12) |
| H(23C) | 1344(64) | -1916(60) | -1970(31) | 46(16) |
| H(23B) | 2636(70) | -960(61) | -1721(30) | 52(17) |
| H(23A) | 1434(53) | -1335(50) | -1244(26) | 30(13) |
| H(24B) | 426(65) | 1058(64) | -2935(29) | 47(16) |
| H(24A) | 1987(64) | 381(57) | -2768(27) | 43(15) |
| H(24C) | 524(60) | -487(60) | -3031(28) | 42(15) |
| H(18A) | 5627(55) | 8146(25) | 961(17) | 385(141) |
| H(18B) | 4979(18) | 9512(60) | 596(7) | 59(18) |
| H(19A) | 2729(43) | 9143(29) | 829(12) | 278(91) |
| H(19B) | 4005(22) | 9038(24) | 1545(23) | 40(14) |
Acylisoselenoureas 7–12
Photoisomerization (a)
Acylselenoureas 1–6 (5 mmol) dissolved in chloroform (30 ml) were irradiated by a filament lamp or by sunlight under an inert gas atmosphere for 12–20 h (course of the isomerization was monitored by TLC). Pure product was obtained after removing of chloroform on an evaporator.
Use of acetic acid (b)
Benzoylselenoureas 1, 3, 5 (5 mmol) dissolved in acetic acid (30 ml) were refluxed for 5–10 min (TLC monitoring). The pure product was obtained after removing of acetic acid on an evaporator.
Controlled melt (c)
Acylselenourea 1–6 (0.5 mmol) was heated on a microscope slide. This slide was placed on the hot–stage of a melting point apparatus. The temperature increase was stopped when it corresponded with the temperature of the exothermic peak on DTA curve. During examination the change in solid character was observed. After coling to sample to a room temperature the mixture of 1–6 and 7–12 was suspended in chloroform, the extract filtered off with silica gel and evaporated.
Ethyl 2–(3–benzoylisoselenoureido)–4,5,6,7–tetrahydrobenzo[b]thiophene–3–carboxylate (7)
M.p. 173–174 °C; Yield (a) 2.13 g (98%), (b) 2.07 (95%), (c) 0.130 (60%); FTIR 3237, 3125 (NH), 1709, 1275 (COOC), 1650 (NCO), 1624 (C=N) cm-1; 1H NMR (CDCl3) 0.96 (3H, t, J 7.1 Hz, OCH2CH3), 1.72–1.76 (4H, m, 5–CH2 and 6–CH2), 2.66 (2H, t, J 5.0 Hz, 4–CH2), 2.67 (2H, t, J 6.0 Hz, 7–CH2), 3.78 (2H, q, J 7.1 Hz, OCH2CH3), 7.50–8.45 (5H, m, C6H5), 12.22 (1H, s, H(N–6)); 13C NMR (CDCl3) 14.15 (CH3, OCH2CH3), 22.74 (C–5), 22.90 (C–6), 24.50 (C–4), 26.28 (C–7), 60.45 (CH2, OCH2CH3), 113.88 (C–3), 128.26 (C–3' and C–5', C6H5), 128.95 (C–4, thiophene), 130.66 (C–2' and C–6', C6H5), 131.64 (C–5, thiophene), 132.65 (C–4', C6H5), 135.35 (C–1', C6H5), 146.43 (C–2), 157.12 (C–Se, 1JC,Se 195 Hz), 165.43 (C=O, COOCH2CH3), , 176.99 (C=O, COC6H5); 15N NMR (CDCl3) 135.9 (N–6), 240.2 (N–8); 77Se NMR (CDCl3) 533; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 286/3.96, 324/4.24, 400/4.11.
Ethyl 2–(3–pivaloylisoselenoureido)–4,5,6,7–tetrahydrobenzo[b]thiophene–3–carboxylate (8)
M.p. 156 °C; Yield (a) 2.03 g (98%), (b) 1.97 (95%), (c) 0.135 (65%); FTIR 3282, 3179 (NH), 1711, 1241 (COOC), 1660 (NCO), 1630 (C=N) cm-1; 1H NMR (CDCl3) 1.24 (3H, t, J 7.1 Hz, OCH2CH3), 1.37 (9H, s, CMe3), 1.76–1.78 (4H, m, 5–CH2 and 6–CH2), 2.65 (2H, t, J 5.0 Hz, 4–CH2), 2.72 (2H, t, J 6.0 Hz, 7–CH2), 4.22 (2H, q, J 7.1 Hz, OCH2CH3), 11.95 (1H, s, H(N–6)); C NMR (CDCl3) 14.46 (CH3, OCH2CH3), 22.83 (C–5), 22.96 (C–6), 24.43 (C–4), 26.34 (C–7), 27.60 (CH3, C(CH3)3, 41.53 (C(CH3)3, 60.46 (CH2, OCH2CH3), 113.48 (C–3), 128.64 (C–4, thiophene), 131.48 (C–5, thiophene), 146.64 (C–2), 156.85 (C–Se, 1JC,Se 195 Hz), 165.34 (C=O, COOCH2CH3), 191.40 (C=O, COCMe3); 15N NMR (CDCl3) 133.8 (N–6), 241.4 (N–8); 77Se NMR (CDCl3) 537; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 286/3.98, 322/4.11, 395/4.12.
Ethyl 2–(3–benzoylisoselenoureido)–4,5–dimethylthiophene–3–carboxylate (9)
M.p. 175–178 °C; Yield (a) 2.00 g (98%), (b) 1.92 (96%), (c) 0.131 (64%); FTIR 3316, 3199 (NH), 1670, 1259 (COOC), 1655 (NCO), 1625 (C=N) cm-1; 1H NMR (CDCl3) 1.00 (3H, t, J 7.1 Hz, OCH2CH3), 2.17 (3H, s, CH3, C–4 thiophene), 2.30 (3H, s, CH3, C–5 thiophene), 3.85 (2H, q, J 7.1 Hz, OCH2CH3), 7.52–8.47 (5H, m, C6H5), 12.23 (1H, s, H(N–6)); 13C NMR (CDCl3) 12.50 (CH3, C–4 thiophene), 14.13 (CH3, OCH2CH3), 14.27 (CH3, C–5 thiophene), 60.60 (CH2, OCH2CH3), 115.05 (C–3), 125.59 (C–4), 128.30 (C–3' and C–5', C6H5), 129.88 (C–5), 130.68 (C–2' and C–6', C6H5), 132.70 (C–4', C6H5), 135.37 (C–1', C6H5), 145.59 (C–2), 157.09 (C–Se), 165.48 (C=O, COOCH2CH3), 177.05 (C=O, COC6H5); UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 282/3.97, 322/4.27, 400/4.13.
Ethyl 2–(3–pivaloylisoselenoureido)–4,5–dimethylthiophene–3–carboxylate (10)
M.p. 185–187 °C; Yield (a) 1.91 g (98%), (b) 1.83 (96%), (c) 0.117 (64%); FTIR 3348, 3189 (NH), 1688, 1260 (COOC), 1652 (NCO), 1625 (C=N) cm-1; 1H NMR (CDCl3) 1.21 (3H, t, J 7.1 Hz, OCH2CH3), 1.36 (9H, s, CMe3), 2.16 (3H, s, CH3, C–4 thiophene), 2.27 (3H, s, CH3, C–5 thiophene), 3.87 (2H, q, J 7.1 Hz, OCH2CH3), 12.09 (1H, s, H(N–6)); UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 270/4.37, 343/4.29, 410/4.21.
Ethyl 2–(3–benzoylisoselenoureido)benzoate (11)
M.p. 108–110 °C; Yield (a) 1.84 g (98%), (b) 1.74 (93%), (c) 0.113 (60%); FTIR 3433, 3181 (NH), 1703, 1256 (COOC), 1650 (NCO), 1625 (C=N) cm-1;1H NMR (CDCl3) 1.03 (3H, t, J 7.1 Hz, OCH2CH3), 3.89 (2H, q, J 7.1 Hz, OCH2CH3), 7.17–8.80 (9H, m, C6H5 and C6H4), 11.40 (1H, s, H(N–6)); 13C NMR (CDCl3) 13.95 (CH3, OCH2CH3), 61.34 (CH2, OCH2CH3), 118.95 (C–1), 124.02 (C–3), 124.11 (C–5), 128.27 (C–3' and C–5', C6H5), 130.21 (C–2' and C–6', C6H5), 130.57 (C–6), 132.60 (C–4', C6H5), 135.66 (C–1', C6H5), 140.61 (C–2), 160.46 (C–Se, 1JC,Se 192 Hz), 166.77 (C=O, COOCH2CH3), 176.83 (C=O, COC6H5), .77Se NMR (CDCl3) 544; UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 270/4.34, 349/4.05, 397/4.64.
Ethyl 2–(3–pivaloylisoselenoureido)benzoate (12)
M.p. 110–112 °C; Yield (a) 1.74 g (98%), (b) 1.60 (90%), (c) 0.108 (61%); FTIR 3436, 3228, 3182 (NH), 1701, 1256 (COOC), 1652 (NCO), 1624 (C=N) cm-1; 1H NMR (CDCl3) 1.27 (9H, s, CMe3), 1.36 (3H, t, J 7.1 Hz, OCH2CH3), 4.44 (2H, q, J 7.1 Hz, OCH2CH3), 7.13–7.99 (4H, m, C6H4), 12.49 (1H, s, H(N–6)); UV–VIS (CHCl3, λmax, nm/log ε, log (m2 mol-1)) 270/3.95, 348/4.15, 396/4.52.