Activity of Alkaloids on Peptic Ulcer: What’s New?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rutaecarpine

2.2. Phenylquinoline

2.3. Nicotine

2.4. Rohitukine

2.5. Methoxycanthin-6-one

2.6. Chelerythrine

2.7. Piplartine

2.8. N-Isopropylmethylanthranilate

2.9. N-Methylmethylanthranilate

2.10. (+)-O-Methylarmepavine

2.11. N-Methylcorydaldine

2.12. Isocorydine

2.13. Canthin-6-one

2.14. Peganine and Derivatives

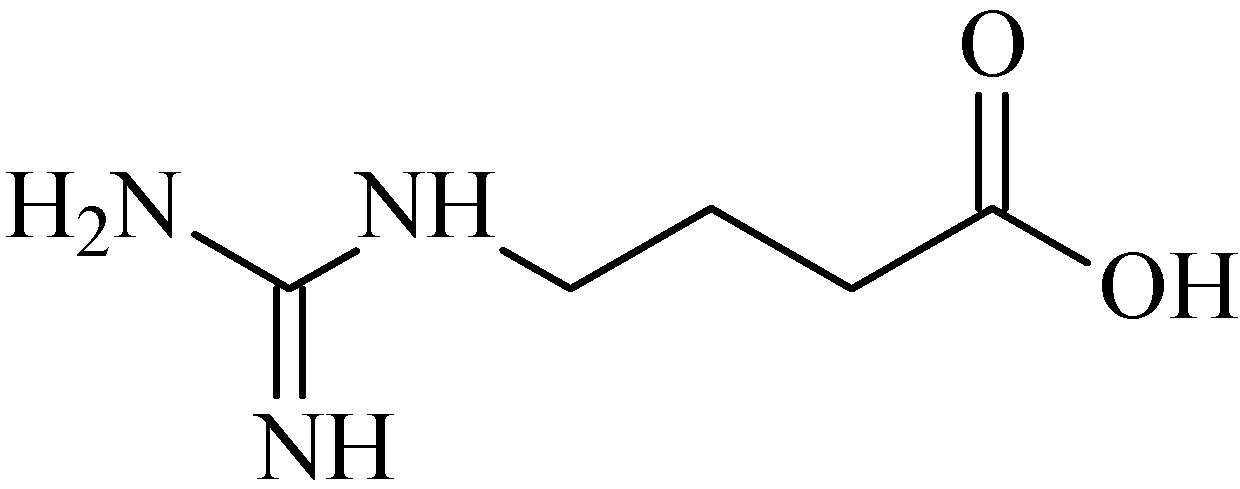
2.15. 4-Guanidinobutyric Acid
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Najim, W.I. Peptic ulcer disease. Prim. Care Clin. 2012, 38, 383–394. [Google Scholar] [CrossRef]
- Nieto, Y.B. Úlcera péptica. Medicine 2012, 11, 137–141. [Google Scholar]
- Lau, J.Y.; Sung, J.; Hill, C.; Henderson, C.; Howden, C.W.; Metz, D.C. Systematic review of the epidemiology of complicated peptic ulcer disease: Incidence, recurrence, risk factors and mortality. Digestion 2011, 84, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Groenen, M.J.M.; Kuipers, E.J.; Hanses, B.E.; Ouwendijk, R.J.T. Incidence of duodenal ulcers and gastric ulcers in a Western population: Back to where it started. Can. J. Gastroenterol. 2009, 23, 604–608. [Google Scholar] [PubMed]
- Harold, K.; Grant, D.; Mitchel, J. Pharmacotherapy of acid peptic disorders. In Principles of Medical Pharmacology, 7th ed.; Elsevier: Toronto, ON, Canada, 2007; pp. 558–559. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Tarnawski, A.; Ahluwalia, A.; Jones, M.K. Gastric cytoprotection beyond prostaglandins: Cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antiacids. Curr. Pharm. Des. 2013, 19, 126–132. [Google Scholar]
- Schmitt, E.K.; Moore, C.M.; Krastel, P.; Petersen, F. Natural products as catalysts for innovation: A pharmaceutical industry perspective. Curr. Opin. Chem. Biol. 2011, 15, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Alkaloids. In Natural Products, 2nd ed.; John Wiley & Sons: Chichester, UK, 2002; pp. 1–512. [Google Scholar]
- Aniszewski, T. Definition, typology and occurrence of alkaloids. In Alkaloids—Secrets of Life, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 1–316. [Google Scholar]
- El-Shazly, A.; Wink, M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Tohme, R.; Darwiche, N.; Gali-Muhtasib, H. A journey under the sea: The quest for marine anti-cancer alkaloids. Molecules 2011, 16, 9665–9696. [Google Scholar] [CrossRef] [PubMed]
- Berdai, M.A.; Labib, S.; Chetouani, K.; Harandou, M. Atropa Belladonna intoxication: A case report. Pan Afr. Med. J. 2012, 11, 72. [Google Scholar] [PubMed]
- Melendez-Camargo, M.E.; Contreras-León, I.; Silva-Torres, R. Diuretic effect of alkaloids fraction extracted from Selaginella lepidophylla (Hook. et Grev.) Spring. Bol. Latinoam. Caribe Plantas 2014, 11, 92–99. [Google Scholar]
- Simpson, L.L. An analysis of the sympathomimetic activity of 6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (TIQ). J. Pharmacol. Exp. Ther. 1975, 192, 365–371. [Google Scholar] [PubMed]
- Orhana, I.; Ozçelik, B.; Karaoğlu, T.; Sener, B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Z. Naturforschung C 2007, 62, 19–26. [Google Scholar]
- Awaad, A.S.; Maitland, D.J.; Moneir, S.M. New alkaloids from Casimiroa edulis fruits and their pharmacological activity. Chem. Nat. Compd. 2007, 43, 576–580. [Google Scholar] [CrossRef]
- Hayfaa, A.A.; Sahar, A.M.; Awatif, M.A. Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. J. Pain Res. 2013, 31, 611–615. [Google Scholar] [CrossRef]
- Nesterova, Y.V.; Povetieva, T.N.; Suslov, N.I.; Semenov, A.A.; Pushkarskiy, S.V. Antidepressant activity of diterpene alkaloids of Aconitum baicalense Turcz. Bull. Exp. Biol. Med. 2011, 151, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Dzhakhangirov, F.N.; Bessonova, I.A. Alkaloids of Aconitum coreanum. X. Curare-like activity-structure relationship. Chem. Nat. Compd. 2002, 38, 74–77. [Google Scholar] [CrossRef]
- Karou, D.; Savadogo, A.; Canini, A.; Yameogo, S.; Montesano, C.; Simpore, J.; Traore, A.S. Antibacterial activity of alkaloids from Sida acuta. Afr. J. Biotechnol. 2006, 5, 195–200. [Google Scholar]
- Malhotra, C.L.; Sidhu, R.K. The anti-emetic activity of alkaloids of Rauwolfia serpentina. J. Pharmacol. Exp. Ther. 1956, 116, 123–129. [Google Scholar] [PubMed]
- Almeida, R.N.; Navarro, D.S.; Barbosa-Filho, J.M. Plants with central analgesic activity. Phytomedicine 2001, 8, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.; Moura, M.D.; Oliveira, R.A.G.; Diniz, M.F.F.; Barbosa-Filho, J.M. Natural product inhibitors of ovarian neoplasia. Phytomedicine 2003, 10, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.G.; Almeida, J.R.G.S.; Macedo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine 2005, 12, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Quintans Júnior, L.J.; Almeida, J.R.G.S.; Lima, J.T.; Nunes, X.P.; Siqueira, J.S.; Oliveira, L.E.G.; Almeida, R.N.; Athayde-Filho, P.F.; Barbosa-Filho, J.M. Plants with anticonvulsant properties—A review. Rev. Bras. Farmacogn. 2008, 18, 798–819. [Google Scholar] [CrossRef]
- Sousa, F.C.F.; Melo, C.T.V.; Citó, M.C.O.; Félix, F.H.C.; Vasconcelos, S.M.M.; Fonteles, M.M.F.; Barbosa Filho, J.M.; Viana, G.S.B. Plantas medicinais e seus constituintes bioativos: Uma revisão da bioatividade e potenciais benefícios nos distúrbios da ansiedade em modelos animais. Rev. Bras. Farmacogn. 2008, 18, 642–654. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Alencar, A.A.; Nunes, X.P.; Tomaz, A.C.A.; Sena-Filho, J.G.; Athayde-Filho, P.F.; Silva, M.S.; Souza, M.F.V.; da-Cunha, E.V.L. Sources of alpha-, beta-, gamma-, delta- and epsilon-carotenes: A twentieth century review. Rev. Bras. Farmacogn. 2008, 18, 135–154. [Google Scholar] [CrossRef]
- Honório Júnior, J.E.R.; Soares, P.M.; Melo, C.L.; Arruda Filho, A.C.V.; Sena Filho, J.G.; Barbosa Filho, J.M.; Sousa, F.C.F.; Fonteles, M.M.F.; Leal, L.K.A.; Queiroz, M.G.R.; et al. Atividade farmacológica da monocrotalina isolada de plantas do gênero Crotalaria. Rev. Bras. Farmacogn. 2010, 20, 453–458. [Google Scholar] [CrossRef]
- Ribeiro Filho, J.; Falcão, H.S.; Batista, L.M.; Barbosa Filho, J.M.; Piuvezam, M.R. Effects of plant extracts on HIV-1 protease. Curr. HIV Res. 2010, 8, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.L.; Silva, M.S.; Tavares, J.F.; Sena-Filho, J.G.; Lucena, H.F.S.; Romero, M.A.V.; Barbosa-Filho, J.M. Tropane alkaloids from genus Erythroxylum: Distribution and compilation of 13C-NMR spectral data. Chem. Biodivers. 2010, 7, 302–326. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.R.M.; Montenegro, C.A.; Almeida, C.L.F.; Athayde-Filho, P.F.; Barbosa-Filho, J.M.; Batista, L.M. Database survey of anti-inflammatory plants in South America: A review. Int. J. Mol. Sci. 2011, 12, 2692–2749. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.L.; Tavares, J.F.; Silva, M.S.; Diniz, M.F.F.M.; Athayde-Filho, P.F.; Barbosa Filho, J.M. Anti-inflammatory activity of alkaloids: An update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.L.; Fischer, D.C.H.; Tavares, J.F.; Silva, M.S.; Athayde-Filho, P.F.; Barbosa-Filho, J.M. Compilation of secondary metabolites from Bidens pilosa L. Molecules 2011, 16, 1070–1102. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.L.F.; Falcão, H.S.; Lima, G.R.M.; Montenegro, C.A.; Lira, N.S.; Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.S.; Bastos, K.X.; Barbosa-Filho, J.M.; Athayde-Filho, P.F.; Diniz, M.F.F.M.; Sobral, M.V. Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid.-Based Complement. Altern. Med 2014. [Google Scholar] [CrossRef]
- Falcão, H.S.; Mariath, I.R.; Diniz, M.F.F.M.; Batista, L.M.; Barbosa-Filho, J.M. Plants of the American continent with antiulcer activity. Phytomedicine 2008, 15, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Mota, K.S.L.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, A.; Souza-Brito, A.R.M.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef] [PubMed]
- Jesus, N.Z.T.; Falcão, H.S.; Gomes, I.F.; Leite, T.J.A.; Lima, G.R.M.; Barbosa-Filho, J.M.; Tavares, J.F.; Silva, M.S.; Athayde-Filho, P.F.; Batista, L.M. Tannins, peptic ulcers and related mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef] [PubMed]
- Falcão, H.S.; Leite, J.A.; Barbosa-Filho, J.M.; Athayde-Filho, P.F.; Chaves, M.C.O.; Moura, M.D.; Ferreira, A.L.; Almeida, A.B.A.; Souza-Brito, A.R.M.; Diniz, M.F.F.M.; et al. Gastric and duodenal antiulcer activity of alkaloids: A review. Molecules 2008, 13, 3198–3223. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Changping, H. Pharmacological effects of rutaercapine as a cardiovascular protective agent. Molecules 2010, 15, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Son, J.K.; Jeong, B.S.; Jeong, T.C.; Chang, H.W.; Lee, E.S.; Jahng, Y. Progress in the studies on rutaecarpine. Molecules 2008, 13, 272–300. [Google Scholar]
- Chavan, S.P.; Sivappa, R. A facile total synthesis of rutaecarpine. Tetrahedron Lett. 2004, 45, 997–999. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.P.; Deng, P.Y.; Shen, S.S.; Zhu, H.Q.; Ding, J.S.; Tan, G.S.; Li, Y.J. The protective effects of rutaecarpine on gastric mucosa injury in rats. Planta Med. 2005, 71, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, P.T.; Carr, A. Gastric acid and digestive physiology. Surg. Clin. N. Am. 2011, 91, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Heeba, G.H.; Abdelwahab, S.A.; Rofaeil, R.R. Protective effects of nebivolol against cold restraint stress-induced gastric ulcer in rats: Role of NO, HO-1, and COX-1,2. Nitric Oxide 2012, 27, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.J.; Liu, B.; Dai, Z.; Yang, Z.C.; Peng, J. Stimulation of calcitonin gene-related peptide release through targeting capsaicin receptor: A potencial strategy for gastric mucosal protection. Dig. Dis. Sci. 2013, 58, 320–325. [Google Scholar] [PubMed]
- Aizawa, H.; Takata, S.; Shigyo, M.; Matsumoto, K.; Inoue, H.; Hara, N. N-omega-nitro-l-arginine methyl ester increases airway responsiveness to serotonin but not to acetylcholine in cats in vivo. Respiration 2001, 68, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Zhou, Y.; Li, D.; Wang, L.; Hu, G.Y.; Peng, J.; Li, Y.J. Reduction of asymmetric dimethylarginine in the protective effects of rutaecarpine on gastric mucosal injury. Can. J. Physiol. Pharmacol. 2008, 86, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, F.; Gandolfi, R.B.; Lemos, M.; Ticona, J.C.; Gimenez, A.; Clasen, B.K.; Filho, V.C.; Andrade, S.F. Gastroprotective activity of alkaloid extract 2-phenylquinoline obtained from the bark of Galipea longiflora Krause (Rutaceae). Chem. Biol. Interact. 2009, 180, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Calla-Margarinos, J.; Giménez, A.; Troye-Blomberg, M.; Fernández, C. An alkaloid extract of evant, traditionally used as anti-leishmania agente in Bolivia, inhibits cellular proliferation and interferon-γ production in polyclonally activated cells. Scand. J. Immunol. 2009, 69, 251–258. [Google Scholar]
- Campos-Buzzi, F.; Fracasso, M.; Clasen, B.K.; Ticona, J.C.; Gimenez, A.; Cechinel-Filho, V. Evaluation of antinoceptive effects of Galipea longiflora alkaloid extract and major alkaloid 2-phenylquinoline. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, H.; Niu, X.; Fan, T.; Mu, Q.; Li, H. Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol. Appl. Pharm. 2013, 272, 21–29. [Google Scholar] [CrossRef]
- Brzozowski, T. Experimental production of peptic ulcer, gastric damage and cancer models and their use in pathophysiological studies and pharmacological treatment—Polish achievements. J. Physiol. Pharmacol. 2003, 54, 99–126. [Google Scholar] [PubMed]
- Sobhian, B.; Jafarmadar, M.; Redl, H.; Bahrami, S. Nitric oxide-supplemented resuscitation improves early gastrointestinal blood flow in rats subjected to hemorrhagic shock without late consequences. Am. J. Surg. 2011, 201, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Kochar, N.I.; Chandewal, A.V.; Bakal, R.L.; Kochar, P.N. Nitric oxide and the gastrointestinal tract. Int. J. Pharmacol. 2011, 7, 31–39. [Google Scholar] [CrossRef]
- Baidoo, E.E.K.; Clench, M.R.; Smith, R.F.; Tetler, L.W. Determination of nicotine and its metabolites in urine by solid-phase extraction and sample stacking capillary electrophoresis-mass spectrometry. J. Chromatogr. 2003, 796, 303–313. [Google Scholar]
- Fallone, C.A.; Morris, G.P. Topical nicotine protects rat gastric mucosa against ASA-induced damage. Dig. Dis. Sci. 1995, 40, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y.; Zou, Y.Y.; Wang, L.; Yang, C.Z.; Guo, R.; Li, D.; Peng, J.; Li, Y.J. Detrimental effects of nicotine on the acute gastric mucosal injury induced by ethanol: Role of asymmetric dimethyllarginine. Can. J. Physiol. Pharmacol. 2008, 86, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.G.; Kattige, S.L.; Bhat, S.V.; Alreja, B.; Souza, N.J.; Rupp, R.H. An antiinflammatory cum immunomodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: Isolation, structure and total synthesis. Tetrahedron 1988, 44, 2081–2086. [Google Scholar] [CrossRef]
- Harmon, A.D.; Weiss, U.; Silverton, J.V. The structure of rohitukine, the main alkaloid of Amoora rohituka (Syn. Aphanamixis polystachya) (Meliaceae). Tetrahedron Lett. 1979, 20, 721–724. [Google Scholar] [CrossRef]
- Yang, D.H.; Cai, S.Q.; Zhao, Y.Y.; Liang, H. A new alkaloid from Dysoxylum binectariferum. J. Asian Nat. Prod. Res. 2004, 6, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Keshri, G.; Oberoi, R.M.; Lakshmi, V.; Pandey, K.; Singh, M.M. Contraceptive and hormonal properties of the stem bark of Dysoxylum binectariferum in rat and docking analysis of rohitukine, the alkaloid isolated from active chloroform soluble fraction. Contraception 2007, 76, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.; Pandey, K.; Kapil, A.; Singh, N.; Samant, M.; Dube, A. In vitro and in vivo leishmanicidal activity of Dysoxylum binectariferum and its fractions against Leishmania donovani. Phytomedicine 2007, 14, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, P.; Shrivastva, S.; Mishra, S.K.; Lakshmi, V.; Sharma, R.; Palit, G. Gastroprotective effect of anti-cancer compound rohitukine: Possible role of gastrin antagonism and H+ K+ -ATPase inhibition. Naunyn. Schmiedebergs. Arch. Pharmacol. 2012, 385, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.L. Gastric exocrine and endocrine secretion. Curr. Opin. Gastroenterol. 2009, 25, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Cell Signalling Biology—Module 7. 2012, pp. 12–24. Available online: http://www.cellsignallingbiology.org (accessed on 8 September 2014). [CrossRef]
- Chu, S.; Schubert, M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2012, 28, 597–593. [Google Scholar] [CrossRef]
- Njar, V.C.O.; Alao, T.O.; Okogun, J.I.; Holland, H.L. 2-Methoxy canthin-6-one: A new alkaloid from the stem wood of Quassia amara. Planta Med. 1993, 59, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Raji, Y.; Bolarinwa, A.F. Antifertility activity of Quassia amara in male rats: In vivo study. Life Sci. 1997, 61, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Raji, Y.; Oloyede, G.K. Antiulcerogenic effects and possible mechanism of action of Quassia amara (L. Simaroubaceae) extract and its bioactive principles in rats. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 112–119. [Google Scholar] [PubMed]
- Stewart, D.J.; Ackroyd, R. Peptic ulcers and their complications. Surgery 2011, 29, 568–574. [Google Scholar]
- Li, W.F.; Hao, D.J.; Fan, T.; Huang, H.M.; Yao, H.; Niu, X.F. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem. Biol. Interact. 2014, 208, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Walterova, D.; Lrichova, J.U.; Valka, I.; Vicar, J.; Vavreckova, C.; Taborska, E.; Harkrader, R.J.; Meyer, D.L.; Cerna, H.; Simanek, V. Benzo[c]phenanthridine alkaloids sanguinarine and CHE: Biological activities and dental care applications. Acta Univ. Palacki. Olomuc. Fac. Medicae 1995, 139, 7–16. [Google Scholar]
- Vogt, A.; Tamewitz, A.; Skoko, J.; Sikorski, R.P.; Giuliano, K.A.; Lazo, J.S. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J. Biol. Chem. 2005, 280, 19078–19086. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Andus, T. Cytokines in inflammatory bowel disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.L.; Raghavendran, H.R.B.; Sung, N.Y.; Kim, J.H.; Chun, B.S.; Ahn, D.H.; Choi, H.S.; Kang, K.W.; Lee, J.W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 83, 249–254. [Google Scholar] [CrossRef]
- Anrather, J.; Csizmadia, V.; Soares, M.P.; Winkler, H. Regulation of NF-kappaB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Czeta in primary endothelial cells. J. Biol. Chem. 1999, 274, 13594–13603. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Fan, N.C.; Lin, M.H.; Chu, L.R.; Huang, S.J.; Hu, C.Y.; Han, S.Y. Antiinflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; Moraes, M.O.; Neto, N.S.; Silveira, E.R.; Lotufo, L.V.C. Overview of the therapeutic potencial of piplastine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Golovine, K.V.; Makhov, P.B.; Teper, E.; Kutikov, A.; Canter, D.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. Prostate 2013, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Vasconcellos, M.C.; Machado, M.S.; Villela, I.V.; Rosa, R.M.; Moura, D.J.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.; et al. Piplartine induces genotoxicity in eukaryotic but not in prokaryotic model systems. Mutat. Res. 2009, 677, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.V.; Lanznaster, D.; Balbinot, D.T.L.; Gadotti, V.M.; Facundo, V.A.; Santos, A.R. Antinociceptive effect of crude extract, fractions and three alkaloids obtained from fruits of Piper tuberculatum. Biol. Pharm. Bull. 2009, 32, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Felipe, F.C.B.; Sousa-Filho, J.T.; Souza, L.E.O.; Silveira, J.A.; Uchoa, D.E.A.; Silveira, E.R.; Pessoa, O.D.L.; Viana, G.S.B. Piplartine, an amide alkaloid from Piper tuberculatum, presents anxiolytic and antidepressant effects in mice. Phytomedicine 2007, 14, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Kim, S.Y.; Han, S.S.; Kim, C.W.; Kumar, S.; Park, B.S.; Lee, S.E.; Yun, Y.P.; Jo, H.; Park, Y.H. Piperlongumine inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochem. Biophys. Res. Commun. 2012, 427, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Muthenna, P.; Shankaraiah, G.; Akileshwari, C.; Babu, K.H.; Suresh, G.; Babu, K.S.; Kumar, R.S.C.; Prasad, K.R.; Yadav, P.A.; et al. Synthesis and biological evaluation of new piplartine analogues as potent aldose reductase inhibitors (ARIs). Eur. J. Med. Chem. 2012, 57, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Naika, R.; Prasanna, K.P.; Ganapathy, P.S.S. Antibacterial activity of piperlongumine an alkaloid isolated from methanolic root extract of Piper longum L. Pharmacophore 2010, 1, 141–148. [Google Scholar]
- Bodiwala, H.S.; Singh, G.; Singh, R.; Dey, C.S.; Sharma, S.S.; Bhutani, K.K.; Singh, I.P. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J. Nat. Med. 2007, 61, 418–421. [Google Scholar] [CrossRef]
- Cotinguiba, F.; Regasini, L.O.; Bolzani, V.S.; Debonsi, H.M.; Passerini, G.D.; Cicarelli, R.M.B.; Kato, M.J.; Furlan, M. Piperamides and their derivatives as potential anti-trypanosomal agents. Med. Chem. Res. 2009, 18, 703–711. [Google Scholar] [CrossRef]
- Moraes, J.; Nascimento, C.; Lopes, P.O.; Nakano, E.; Yamaguchi, L.F.; Kato, M.J.; Kawano, T. Schistosoma mansoni: In vitro schistosomicidal activity of piplartine. Exp. Parasitol. 2011, 127, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Burci, L.M.; Pereira, I.T.; Silva, L.M.; Rodrigues, R.V.; Facundo, V.A.; Militão, J.S.L.T.; Santos, A.R.S.; Marques, M.C.A.; Baggio, C.H.; Werner, M.F.P. Antiulcer and gastric antisecretory effects of dichloromethane fraction and piplartine obtained from fruits of Piper tuberculatum Jacq. in rats. J. Ethnopharmacol. 2013, 148, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Whittle, B.J. Gastrointestinal effects of nonsteroidal anti-inflammatory drugs. Fundam. Clin. Pharm. 2003, 17, 301–313. [Google Scholar] [CrossRef]
- Repetto, M.G.; Llesuy, S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res. 2002, 35, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Cnubben, N.H.P.; Rietjens, I.M.C.M.; Wortelboer, H.; VanZanden, J.; VanBladeren, P.J. The inter play of glutathione-related processes in antioxidant defense. Environ. Toxicol. Pharm. 2001, 10, 141–152. [Google Scholar] [CrossRef]
- Radulović, N.S.; Miltojević, A.B.; McDermott, M.; Waldren, S.; Parnellc, J.A.; Pinheiro, M.M.G.; Fernandes, P.D.; Menezes, F.S. Identification of a new antinociceptive alkaloid isopropyl N-methylanthranilate from the essential oil of Choisya ternataKunth. J. Ethnopharmacol. 2011, 135, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Miltojević, A.B.; Randjelović, P.J.; Stojanović, N.M.; Boylan, F. Effects of methyl and isopropyl N-methylanthranilates from Choisya ternata Kunth (Rutaceae) on experimental anxiety and depression in mice. Phytother. Res. 2013, 27, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Jovanović, I.; Ilić, I.R.; Randjelović, P.J.; Stojanović, N.M.; Miltojević, A.B. Methyl and isopropyl N-methylanthranilates attenuate diclofenac- and ethanol-induced gastric lesions in rats. Life Sci. 2013, 93, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Bhakuni, D.S.; Tewari, S.; Dhar, M.M. Aporphine alkaloids of Annona squamosa. Phytochemistry 1972, 11, 1819–1822. [Google Scholar] [CrossRef]
- Venkov, A.P.; Statkova-Abeghe, S.M. Synthesis of 3,4-dihydroisoquino-lines, 2-alkyl (Acyl)-1(2 H)-3,4-dihydroisoquinolinones, 2-alkyl-1(2 H)-isoquinolinones and 1-alkyl-2(2 H)-quinolinones by oxidation with potassium permanganate. Tetrahedron 1996, 52, 1451–1460. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Moriyasu, M.; Ichimaru, M.; Iwasa, K.; Kato, A.; Mathenge, S.G.; Mutiso, P.B.C.; Juma, F.D. Secondary and tertiary isoquinoline alkaloids from Xylopia parviflora. Phytochemistry 2006, 67, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Istatkova, R.; Nikolaeva-Glomb, L.; Galabov, A.; Yadamsuren, G.O.; Samdan, J.; Dangaa, S.; Philipov, S. Chemical and antiviral study on alkaloids from Papaver pseudocanescens M. Pop. Z. Naturforschung C 2012, 67, 22–28. [Google Scholar] [CrossRef]
- Vila-Nova, N.S.; Morais, S.M.; Falcão, M.J.C.; Machado, L.K.A.; Beviláqua, C.M.L.; Costa, I.R.S.; Brasil, N.V.G.P.S.; Júnior, H.F.A. Leishmanicidal activity and cytotoxicity of compounds from two Annonacea species cultivated in Northeastern Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44, 567–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, V.K.; Yadav, D.K.; Bano, N.; Dixit, P.; Pathak, M.; Maurya, R.; Sahai, M.; Jain, S.K.; Misra-Bhattacharya, S. N-methyl-6,7-dimethoxyisoquinolone in Annona squamosa twigs is the major immune modifier to elicit polarized Th1 immune response in BALB/c mice. Fitoterapia 2012, 83, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Singh, N.; Dev, K.; Sharma, R.; Sahai, M.; Palit, G.; Maurya, R. Anti-ulcer constituents of Annona squamosa twigs. Fitoterapia 2011, 82, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.S.S.; Filho, V.C.; Niero, R.; Clasen, B.K.; Balogun, S.O.; Oliveira Martins, D.T. Pharmacological mechanisms underlying the anti-ulcer activity of metanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models. J. Ethnopharmacol. 2011, 134, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Benkrief, R.; Brum-Bousquet, M.; Tillequin, F.; Koch, M. Alkaloids and flavonoid from aerial parts of Hammada articulata ssp. scoparia. Ann. Pharm. Fr. 1990, 48, 219–224. [Google Scholar] [PubMed]
- Sener, B.; Gozler, B.; Minard, R.D.; Shamma, M. Alkaloids of Fumarza villantii. Phytochemistry 1983, 22, 2073–2075. [Google Scholar] [CrossRef]
- Gu, J.Q.; Park, E.J.; Totura, S.; Riswan, S.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of the twigs of Hernandia ovigera that inhibit the transformation of JB6 murine epidermal cells. J. Nat. Prod. 2002, 65, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Istatkova, R.S.; Philipov, S.A. Alkaloids from Isopyrum thalictroides L. Phytochemistry 2000, 54, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.Q.; Yang, Y.F.; Ai, T.M. Determination of protopine and isocorydine in root of Dactylicapnos scandens by HPLC. Zhongguo Zhong Yao Za Zhi 2004, 29, 961–963. [Google Scholar] [PubMed]
- Cheng, X.; Wang, D.; Jiang, L.; Yang, D. Simultaneous determination of eight bioactive alkaloids in Corydalis saxicola by high-performance liquid chromatography coupled with diode array detection. Phytochem. Anal. 2008, 19, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.S.; Davis, R.A.; Duffy, S.; Avery, V.M.; Quinn, R.J. Antimalarial benzylisoquinoline alkaloid from the rainforest tree Doryphora sassafras. J. Nat. Prod. 2009, 72, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Song, Y.; Chen, D.; Xue, P.; Tu, P.; Wang, K.; Chen, J. Studies on chemical constituents of leaves of Aquilaria sinensis. Zhongguo Zhong Yao Za Zhi 2009, 34, 858–860. [Google Scholar] [PubMed]
- He, L.; Zhang, Y.; Tang, L.; Song, S.; Sun, Q. Alkaloids in stems and leaves of Stephania cepharantha. Zhongguo Zhong Yao Za Zhi 2010, 35, 1272–1275. [Google Scholar] [PubMed]
- Wang, H.Y.; Zuo, A.X.; Sun, Y.; Rao, G.X. Chemical constituents of Aconitum brachypodum from Dong-Chuan area. Zhongguo Zhong Yao Za Zhi 2013, 38, 4324–4328. [Google Scholar] [PubMed]
- Chen, Z.; Zhang, Z.; Wang, M. Spasmolytic effects of isocorydine on isolated gall-bladder and oddi’s sphincter in vitro. Acta Pharm. Sin. 1985, 6, 45–48. [Google Scholar]
- Lu, P.; Sun, H.; Zhang, L.; Hou, H.; Zhang, L.; Zhao, F.; Ge, C.; Yao, M.; Wang, T.; Li, J. Isocorydine targets the drug-resistant cellular side population through PDCD4-related apoptosis in hepatocellular carcinoma. Mol. Med. 2012, 18, 1136–1146. [Google Scholar] [CrossRef]
- Sun, H.; Hou, H.; Lu, P.; Zhang, L.; Zhao, F. Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/M cell cycle arrest and apoptosis. PLoS One 2012, 7, e36808. [Google Scholar] [CrossRef] [PubMed]
- Shahwar, D.; Raza, M.A.; Tariq, S.; Riasat, M.; Ajaib, M. Enzyme inhibition, antioxidant and antibacterial potential of vasicine isolated from Adhatoda vasica Nees. Pak. J. Pharm. Sci. 2012, 25, 651–656. [Google Scholar] [PubMed]
- Malik, J.K.; Sharma, A.; Singh, S.; Jain, S. Nanosuspension of vasicine from Adhatoda vasica: Isolation and characterization. Drug Invent. Today 2013, 5, 32–38. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, V.; Tiwari, S.; Khaliq, T.; Barthwal, M.K.; Pandey, H.P.; Palit, G.; Narender, T. Anti-secretory and cyto-protective effects of peganine hydrochloride isolated from the seeds of Peganum harmala on gastric ulcers. Phytomedicine 2013, 20, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Lamchouri, F.; Zemzami, M.; Jossang, A.; Abdellatif, A.; Israili, Z.H.; Lyoussi, B. Cytotoxicity of alkaloids isolated from Peganum harmala seeds. Pak. J. Pharm. Sci. 2013, 26, 699–706. [Google Scholar] [PubMed]
- Tachikawa, M.; Hosoya, K. Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: Relevance to neural disorders. Fluids Barriers CNS 2011, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.Y.; Jeong, C.S. Inhibitory effects of 4-guanidinobutyric acid against gastric lesions. Biomol. Ther. 2012, 20, 239–244. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do Nascimento, R.F.; De Sales, I.R.P.; De Oliveira Formiga, R.; Barbosa-Filho, J.M.; Sobral, M.V.; Tavares, J.F.; Diniz, M.D.F.F.M.; Batista, L.M. Activity of Alkaloids on Peptic Ulcer: What’s New? Molecules 2015, 20, 929-950. https://doi.org/10.3390/molecules20010929
Do Nascimento RF, De Sales IRP, De Oliveira Formiga R, Barbosa-Filho JM, Sobral MV, Tavares JF, Diniz MDFFM, Batista LM. Activity of Alkaloids on Peptic Ulcer: What’s New? Molecules. 2015; 20(1):929-950. https://doi.org/10.3390/molecules20010929
Chicago/Turabian StyleDo Nascimento, Raphaela Francelino, Igor Rafael Praxedes De Sales, Rodrigo De Oliveira Formiga, José Maria Barbosa-Filho, Marianna Vieira Sobral, Josean Fechine Tavares, Margareth De Fátima Formiga Melo Diniz, and Leônia Maria Batista. 2015. "Activity of Alkaloids on Peptic Ulcer: What’s New?" Molecules 20, no. 1: 929-950. https://doi.org/10.3390/molecules20010929