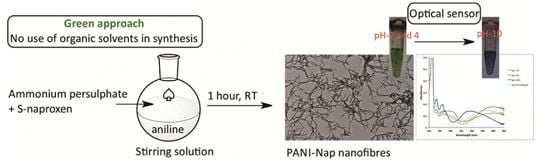
Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization

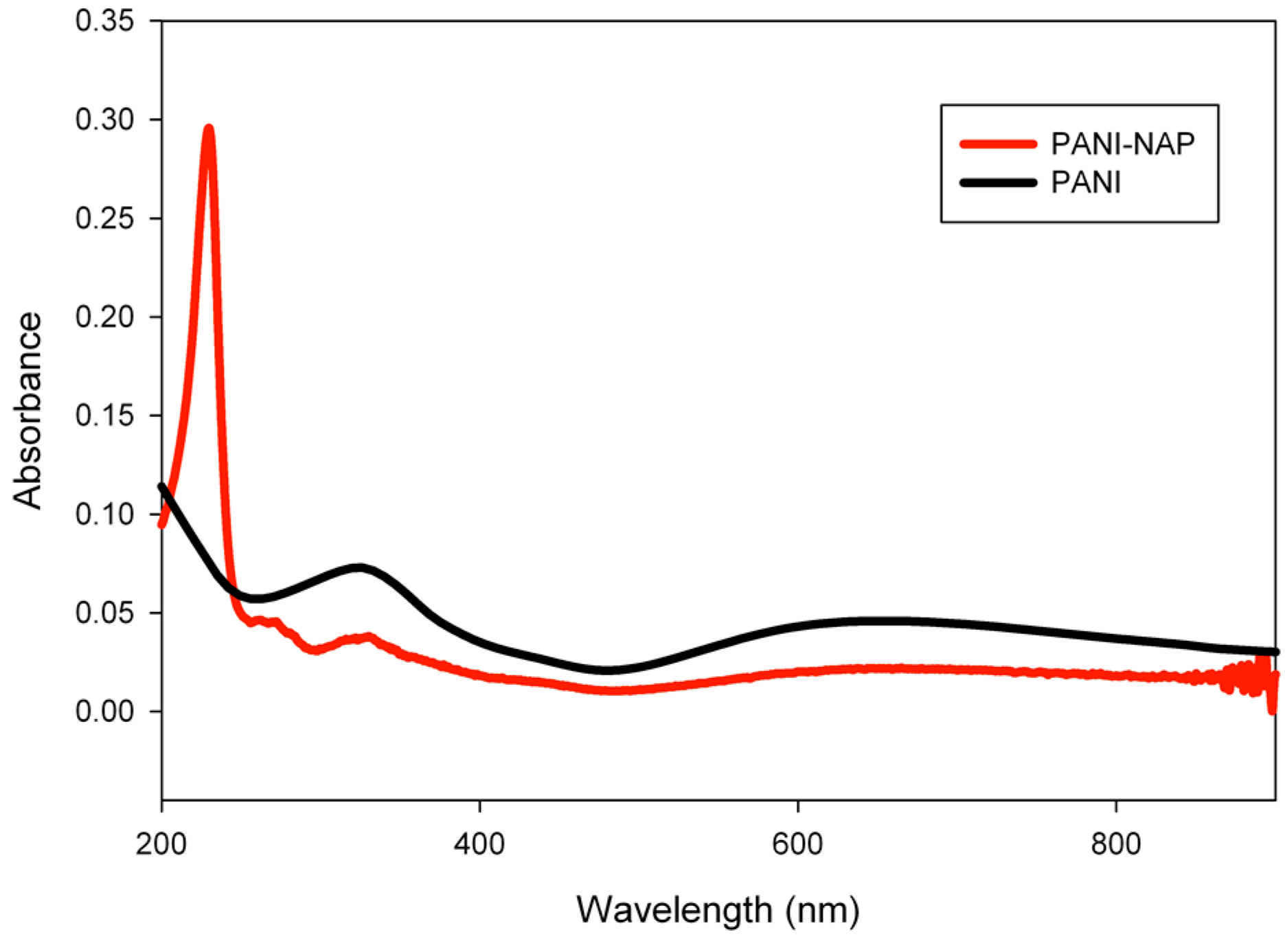




2.2. pH Sensing Application




3. Experimental Section
3.1. Chemicals and Equipment
3.2. Synthesis and Characterization of PANI-NAP
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moutet, J.C.; Saintaman, E.; Tranvan, F.; Angibeaud, P.; Utille, J.P. Poly(glucose-pyrrole) modified electrodes—A novel chiral electrode for enantioselective recognition. Adv. Mater. 1992, 4, 511–513. [Google Scholar] [CrossRef]
- Guo, H.L.; Knobler, C.M.; Kaner, R.B. A chiral recognition polymer based on polyaniline. Synth. Met. 1999, 101, 44–47. [Google Scholar] [CrossRef]
- Tang, B.Z.; Geng, Y.H.; Lam, J.W.Y.; Li, B.S.; Jing, X.B.; Wang, X.H.; Wang, F.S.; Pakhomov, A.B.; Zhang, X.X. Processible nanostructured materials with electrical conductivity and magnetic susceptibility: Preparation and properties of maghemite polyaniline nanocomposite films. Chem. Mater. 1999, 11, 1581–1589. [Google Scholar] [CrossRef]
- Lin, H.K.; Chen, S.A. Synthesis of new water-soluble self-doped polyaniline. Macromolecules 2000, 33, 8117–8118. [Google Scholar] [CrossRef]
- Su, S.J.; Kuramoto, N. Optically active polyaniline derivatives prepared by electron acceptor in organic system: Chiroptical properties. Macromolecules 2001, 34, 7249–7256. [Google Scholar] [CrossRef]
- Wei, X.L.; Wang, Y.Z.; Long, S.M.; Bobeczko, C.; Epstein, A.J. Synthesis and physical properties of highly sulfonated polyaniline. J. Am. Chem. Soc. 1996, 118, 2545–2555. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Egan, V.M.; Guo, H.L.; Yoon, J.Y.; Briseno, A.L.; Rauda, I.E.; Garrell, R.L.; Knobler, C.M.; Zhou, F.M.; Kaner, R.B. Enantioselective discrimination of d- and l-phenylalanine by chiral polyaniline thin films. Adv. Mater. 2003, 15, 1158–1161. [Google Scholar] [CrossRef]
- Schwientek, M.; Pleus, S.; Hamann, C.H. Enantioselective electrodes: Stereoselective electroreduction of 4-methylbenzophenone and acetophenone1. J. Electroanal. Chem. 1999, 461, 94–101. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yashima, E. Polysaccharide derivatives for chromatographic separation of enantiomers. Angew. Chem. Int. Ed. 1998, 37, 1020–1043. [Google Scholar] [CrossRef]
- Yin, X.L.; Ding, J.J.; Zhang, S.; Kong, J.L. Enantioselective sensing of chiral amino acids by potentiometric sensors based on optical active polyaniline films. Biosens. Bioelectron. 2006, 21, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Wan, M.X. Chiral polyaniline nanotubes synthesized via a self-assembly process. Thin Solid Films 2005, 477, 24–31. [Google Scholar] [CrossRef]
- Li, W.G.; Wang, H.L. Oligomer-assisted synthesis of chiral polyaniline nanofibers. J. Am. Chem. Soc. 2004, 126, 2278–2279. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yu, Z.; Huang, Y.W.; Yuan, W.X.; Wei, Z.X. Helical polyaniline nanofibers induced by chiral dopants by a polymerization process. Adv. Mater. 2007, 19, 3353–3357. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.H.; Luo, W.; Liu, Y.; Tang, H.Q. Correlation between one-directional helical growth of polyaniline and its optical activity. J. Phys. Chem. C 2007, 111, 8383–8388. [Google Scholar] [CrossRef]
- Li, W.G.; Wang, H.L. Electrochemical synthesis of optically active polyaniline films. Adv. Funct. Mater. 2005, 15, 1793–1798. [Google Scholar] [CrossRef]
- Hino, T.; Kumakura, T.; Kuramoto, N. Optically active fluoro-substituted polyaniline prepared in organic media: The synthesis, chiroptical properties, and comparison with optically active non-substituted polyaniline. Polymer 2006, 47, 5295–5302. [Google Scholar] [CrossRef]
- Yuan, G.L.; Kuramoto, N. Helical polyaniline induced by specific interaction with biomolecules in neutral solution. Polymer 2003, 44, 5501–5504. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W. Potential controlled electrochemical assembly of chiral polyaniline with enhanced stereochemical selectivity. Polymer 2007, 48, 5473–5479. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Pornputtkul, Y.; Kane-Maguire, L.A.P.; Wallace, G.G. Influence of electrochemical polymerization temperature on the chiroptical properties of (+)-camphorsulfonic acid-doped polyaniline. Macromolecules 2006, 39, 5604–5610. [Google Scholar] [CrossRef]
- Chen, J.; Winther-Jensen, B.; Pornputtkul, Y.; West, K.; Kane-Maquire, L.; Wallace, G.G. Synthesis of chiral polyaniline films via chemical vapor phase polymerization. Electrochem. Solid State 2006, 9, C9–C11. [Google Scholar] [CrossRef]
- Moriuchi, T.; Shen, X.; Hirao, T. Chirality induction of π-conjugated chains through chiral complexation. Tetrahedron 2006, 62, 12237–12246. [Google Scholar] [CrossRef]
- Mire, C.A.; Kane-Maguire, L.A.P.; Wallace, G.G.; Panhuis, M.I.H. Influence of added hydrogen bonding agents on the chiroptical properties of chiral polyaniline. Synth. Met. 2009, 159, 715–717. [Google Scholar] [CrossRef]
- Marinakos, S.M.; Anderson, M.F.; Ryan, J.A.; Martin, L.D.; Feldheim, D.L. Encapsulation, permeability, and cellular uptake characteristics of hollow nanometer-sized conductive polymer capsules. J. Phys. Chem. B 2001, 105, 8872–8876. [Google Scholar] [CrossRef]
- Liang, L.; Liu, J.; Windisch, C.F.; Exarhos, G.J.; Lin, Y. Direct assembly of large arrays of oriented conducting polymer nanowires. Angew. Chem. Int. Ed. 2002, 41, 3665–3668. [Google Scholar] [CrossRef]
- Sudha; Devendra, K.; Mitsumasa, I. Role of saturated solutions of chiral amino acids in synthesis and phase segregation within optically active polyaniline. J. Appl. Polym. Sci. 2013, 127, 3693–3698. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.C.; Feng, W. Chiral polyaniline with flaky, spherical and urchin-like morphologies synthesized in the l-phenylalanine saturated solutions. Synth. Met. 2009, 159, 1597–1602. [Google Scholar] [CrossRef]
- Zeifman, Y.S.; Maiboroda, I.O.; Grishchenko, Y.V.; Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Yaropolov, A.I. Enzymatic synthesis of electroconductive biocomposites based on DNA and optically active polyaniline. Appl. Biochem. Microbiol. 2012, 48, 145–150. [Google Scholar] [CrossRef]
- Li, C.; Yan, J.; Hu, X.J.; Liu, T.; Sun, C.H.; Xiao, S.Z.; Yuan, J.T.; Chen, P.; Zhou, S.Y. Conductive polyaniline helixes self-assembled in the absence of chiral dopant. Chem. Commun. 2013, 49, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, F.; Buschmann, N.; Cammann, K. A fibre-optical sensor for the determination of sodium with a reversible response. Sens. Actuators B Chem. 1992, 9, 41–47. [Google Scholar] [CrossRef]
- DeMarcos, S.; Wolfbeis, O.S. Optical sensing of pH based on polypyrrole films. Anal. Chim. Acta 1996, 334, 149–153. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.X.; Duan, Y.X. An improved optical pH sensor based on polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Ohsaka, T.; Ohnuki, Y.; Oyama, N.; Katagiri, G.; Kamisako, K. IR absorption spectroscopic identification of electroactive and electroinactive polyaniline films prepared by the electrochemical polymerization of aniline. J. Electroanal. Chem. 1984, 161, 399–405. [Google Scholar] [CrossRef]
- Bloor, D.; Monkman, A. Spectroscopic studies of polyaniline. Synth. Met. 1987, 21, 175–179. [Google Scholar] [CrossRef]
- Ge, Z.F.; Brown, C.W.; Sun, L.F.; Yang, S.C. Fiberoptic pH sensor-based on evanescent-wave absorption-spectroscopy. Anal. Chem. 1993, 65, 2335–2338. [Google Scholar] [CrossRef]
- Pringsheim, E.; Terpetschnig, E.; Wolfbeis, O.S. Optical sensing of pH using thin films of substituted polyanilines. Anal. Chim. Acta 1997, 357, 247–252. [Google Scholar] [CrossRef]
- Grummt, U.W.; Pron, A.; Zagorska, M.; Lefrant, S. Polyaniline based optical pH sensor. Anal. Chim. Acta 1997, 357, 253–259. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M.; Janata, J. Acid doping of polyaniline: Spectroscopic and electrochemical studies. J. Phys. Chem. B 1999, 103, 10992–10998. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Simon, E. Poly(aniline)-poly(acrylate) composite films as modified electrodes for the oxidation of NADH. Phys. Chem. Chem. Phys. 2000, 2, 2599–2606. [Google Scholar] [CrossRef]
- Raitman, O.A.; Katz, E.; Buckmann, A.F.; Willner, I. Integration of polyaniline/poly(acrylic acid) films and redox enzymes on electrode supports: An in situ electrochemical/surface plasmon resonance study of the bioelectrocatalyzed oxidation of glucose or lactate in the integrated bioelectrocatalytic systems. J. Am. Chem. Soc. 2002, 124, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A.; Bobrova, O.A.; Luckachova, L.V.; Karyakina, E.E. Potentiometric biosensors based on polyaniline semiconductor films. Sens. Actuators B Chem. 1996, 33, 34–38. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Neftyakova, L.V.; Karyakin, A.A. A novel potentiometric glucose biosensor based on polyaniline semiconductor-films. Anal. Lett. 1994, 27, 2871–2882. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 2002, 531, 43–52. [Google Scholar] [CrossRef]
- Malinauskas, A.; Holze, R. Cyclic UV-vis spectrovoltammetry of polyaniline. Synth. Met. 1998, 97, 31–36. [Google Scholar] [CrossRef]
- Chiang, J.C.; Macdiarmid, A.G. Polyaniline-protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Bao-Fena, Y.E.; Zhi-Jiea, Z.; Huang-Xian, J.U. Fluorescence study on the interaction between naproxen and yeast DNA. Chin. J. Chem. 2005, 23, 58–62. [Google Scholar]
- Tanwar, S.; Ho, J.A.A.; Magi, E. Green synthesis and characterization of novel gold nanocomposites for electrochemical sensing applications. Talanta 2013, 117, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.F.; Zhao, K.; Chen, X.; Li, M.Q.; Liu, J.H. Preparation of gold/polyaniline/multiwall carbon nanotube nanocomposites and application in ammonia gas detection. J. Mater. Sci. 2008, 43, 5861–5866. [Google Scholar] [CrossRef]
- Wei, M.; Shi, S.X.; Wang, J.; Li, Y.; Duan, X. Studies on the intercalation of naproxen into layered double hydroxide and its thermal decomposition by in situ FT-IR and in situ HT-XRD. J. Solid State Chem 2004, 177, 2534–2541. [Google Scholar] [CrossRef]
- Yuet, J.; Epstein, A.J. XPS study of self-doped conducting polyaniline and parent systems. Macromolecules 1991, 24, 4441–4445. [Google Scholar]
- Han, M.G.; Im, S.S. X-ray photoelectron spectroscopy study of electrically conducting polyaniline/polyimide blends. Polymer 2000, 41, 3253–3262. [Google Scholar] [CrossRef]
- Zhang, X.T.; Chechik, V.; Smith, D.K.; Walton, P.H.; Duhme-Klair, A.K.; Luo, Y.J. Nanocomposite hydrogels-controlled synthesis of chiral polyaniline nanofibers and their inclusion in agarose. Synth. Met. 2009, 159, 2135–2140. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanwar, S.; Ho, J.-a.A. Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing. Molecules 2015, 20, 18585-18596. https://doi.org/10.3390/molecules201018585
Tanwar S, Ho J-aA. Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing. Molecules. 2015; 20(10):18585-18596. https://doi.org/10.3390/molecules201018585
Chicago/Turabian StyleTanwar, Shivani, and Ja-an Annie Ho. 2015. "Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing" Molecules 20, no. 10: 18585-18596. https://doi.org/10.3390/molecules201018585