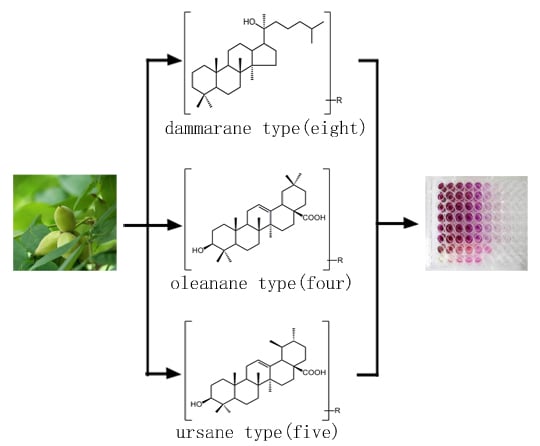
Cytotoxicity of Triterpenes from Green Walnut Husks of Juglans mandshurica Maxim in HepG-2 Cancer Cells
Abstract
:1. Introduction

2. Results and Discussion
2.1. Isolation and Characterization of Compound 6


| No. | δH (J in Hz) | δC (from HSQC) | DEPT | HMBC | COSY |
|---|---|---|---|---|---|
| 1 | 1.48 (m) 1.95 (ddd, 15.8, 9.4, 3.0) | 39.6 | CH2 | C-2, C-3, C-19 | H-2 |
| 2 | 2.45 (ddd, 15.8, 4.5, 3.0) 2.53 (ddd, 15.8, 9.4, 3.0) | 34.1 | CH2 | C-1, C-3, C-10 | H-1 |
| 3 | - | 217.9 | C | ||
| 4 | - | 47.4 | C | ||
| 5 | 1.47 (m) | 55.2 | CH | C-8, C-9, C-19, C-28, C-29 | H-6 |
| 6 | 1.54 (m) | 19.6 | CH2 | C-5, C-9, C-18, C-19 | H-5, H-7 |
| 7 | 1.30 (m) 1.53 (m) | 34.1 | CH2 | C-8, C-18 | H-6 |
| 8 | - | 39.8 | C | ||
| 9 | 1.52 (m) | 49.4 | CH | C-10, C-19 | H-11 |
| 10 | - | 36.8 | C | ||
| 11 | 1.31 (m) 1.82 (m) | 31.7 | CH2 | C-12 | H-9, H-12 |
| 12 | 3.59 (dt, 10.4, 5.1) | 70.8 | CH | C-17 | H-11 |
| 13 | 1.75 (m) | 47.8 | CH | C-8, C-12, C-17 | H-12 |
| 14 | - | 51.6 | C | ||
| 15 | 1.05 (m) 1.51 (m) | 31.0 | CH2 | C-14, C-17 | H-16 |
| 16 | 1.26 (m) 1.88 (m) | 26.6 | CH2 | C-17 | H-15, H-17 |
| 17 | 2.05 (m) | 53.5 | CH | C-13, C-17, C-20, C-22 | H-16 |
| 18 | 0.98 (s) | 16.0 | CH3 | C-7, C-8, C-10, C-14, C-19 | |
| 19 | 1.01 (s) | 15.3 | CH3 | C-5, C-8, C-9, C-10 | |
| 20 | - | 73.6 | C | ||
| 21 | 1.19 (s) | 27.0 | CH3 | C-17, C-20, C-22 | |
| 22 | 1.52 (m) 1.62 (m) | 29.8 | CH2 | C-20, C-23 | H-23 |
| 23 | 1.62 (m) 1.83 (m) | 28.6 | CH2 | C-22 | H-22, H-24 |
| 24 | 4.13 (m) | 75.3 | CH | C-23, C-26 | H-23 |
| 25 | - | 147.4 | C | ||
| 26 | 4.87 (s) 4.98 (s) | 110.6 | CH2 | C-24, C-27 | |
| 27 | 1.73 (s) | 18.6 | CH3 | C-24, C-26 | |
| 28 | 1.08 (s) | 26.7 | CH3 | C-3, C-4, C-5, C-29 | |
| 29 | 1.04 (s) | 21.0 | CH3 | C-3, C-4, C-5, C-28 | |
| 30 | 0.86 (s) | 16.8 | CH3 | C-8, C-14, C-15, C-16, C-18 |
2.2. Cytotoxic Activity
| Comp. | Structural Features | IC50 (μM) a | SD b |
|---|---|---|---|
| PC c | metal complex | 4.61 | 0.66 |
| 1 | dammarane-type | NA | - |
| 2 | NA | - | |
| 3 | 62.23 | 3.06 | |
| 4 | 91.69 | 4.92 | |
| 5 | NA | - | |
| 6 | 58.12 | 4.22 | |
| 7 | 10.32 | 1.13 | |
| 8 | 76.53 | 3.39 | |
| 9 | oleanane-type | 39.42 | 2.55 |
| 10 | 95.5 | 4.46 | |
| 11 | 34.80 | 0.33 | |
| 12 | 16.13 | 3.83 | |
| 13 | ursane-type | 28.69 | 3.17 |
| 14 | NA | - | |
| 15 | 47.22 | 1.98 | |
| 16 | NA | - | |
| 17 | 15.97 | 2.47 |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Cytotoxicity Assays
3.5.1. Cell Culture
3.5.2. Measurement of Cell Proliferation by MTT Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Ma, X.H.; Wang, Z.W. Anticancer drug discovery in the future: An evolutionary perspective. Drug Discov. Today 2009, 14, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.N.; Grace, B.L.; Shafi, G.; Al-Hazzani, A.A.; Alshatwi, A.A. Anti-proliferative effects of organic extracts from root bark of Juglans regia L. (RBJR) on MDA-MB-231 human breast cancer cells: Role of Bcl-2/Bax, caspases and Tp53. Asian-Pac. J. Cancer Prev. 2011, 12, 525–530. [Google Scholar] [PubMed]
- Liu, J.X.; Di, D.L.; Wei, X.N.; Han, Y. Cytotoxic diarylheptanoids from the pericarps of walnuts (Juglans regia). Planta Med. 2008, 74, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Majd, A.; Sepahdar, Z.; Azadmanesh, K.; Irian, S.; Ardestaniyan, M.H.; Hedayati, M.H.; Rastkari, N. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm. Biol. 2012, 50, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M.P. Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm. Biol. 2011, 49, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Fujimoto, Y. 2-Acety-1-(3-glycosyloxyoctadecanoyl) glycerol and dammarane triterpenes in the exudates from glandular trichome-like secretory organs on the stipules and leaves of Cerasus yedoensis. Phytochem. Lett. 2011, 4, 38–42. [Google Scholar] [CrossRef]
- Asakawa, J.; Kasal, R.; Yamasakl, K.; Tanaka, O. 13C-NMR study of ginseng sapogenins and their related dammarane type triterpenes. Tetrahedron 1977, 33, 1935–1939. [Google Scholar] [CrossRef]
- Malinovskaya, G.V.; Novikov, V.L.; Denisenko, V.A.; Uvarova, N.I. A new trirerpene from the leaves of Betula mandschurica. Chem. Nat. Compd. 1980, 16, 257–261. [Google Scholar] [CrossRef]
- Asai, T.; Hara, N.; Fujimoto, Y. Fatty acid derivatives and dammarane triterpenes from the glandular trichome exudates of Ibicella lutea and Proboscidea louisiana. Phytochemistry 2010, 71, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Ideo, R.; Aoki, T.; Suga, T. The structure of anuserrutriol, a new C31 damarane-type triterpenoid from the male flowers of Alnus serrulatoides. Bull. Chem. Soc. Jpn. 1982, 55, 639–640. [Google Scholar] [CrossRef]
- Hou, W.L.; Li, Y.F.; Zhang, Q.; Wei, X.; Peng, A.H.; Chen, L.J.; Wei, Y.Q. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef] [PubMed]
- David, T.; John, W.W. 3-Epikatonic acid from guar meal, Cyamopsis tetragonoloba. Phytochemistry 1980, 19, 1247–1248. [Google Scholar]
- Ramesh, A.S.; Christopher, J.G.; Radhika, R.; Setty, C.R.; Thankamani, V. Isolation, characterisation and cytotoxicity study of arjunolic acid from Terminalia arjuna. Nat. Prod. Res. 2012, 26, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Ibrahim, S.A.; Jalil, S.; Choudhary, M.I. Ursolic Acid: A Potent Inhibitor of Superoxides Produced in the Cellular System. Phytother. Res. 2007, 21, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Echeverri, T.; Rivero-Cruz, J.F.; Su, B.N.; Chai, H.B.; Cordell, G.A.; Pezzuto, J.M.; Swanson, S.M.; Soejarto, D.D.; Kinghorn, A.D. Constituents of the leaves and twigs of Calyptranthes pallens collected from an experimental plot in southern florida. J. Nat. Prod. 2005, 68, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Fourie, T.G.; Matthee, E.; Snyckers, F.O. A pentacyclic triterpene acid, with anti-ulcer properties, from Cussonia natalensis. Phytochemistry 1989, 28, 2851–2852. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xiang, L.M.; Chen, M.; Zhang, Z.X.; He, X.J. Substrate specificity for the 2 α-hydroxylation of ursolic acid by Alternaria alternata and the antitumor activities of those metabolites. J. Mol. Catal. B Enzym. 2012, 83, 51–56. [Google Scholar] [CrossRef]
- Ren, S.; Meng, L.; Wang, G.H.; Zhao, J.L.; He, B.F. Drug cocktail therapy of traditional Chinese medicine in the Clinical and immunological research of the treatment of liver cancer. Inform. Tradit. Chin. Med. 2000, 18, 19–20. [Google Scholar]
- Eidi, A.; Moghadam, J.Z.; Mortazavi, P.; Rezazadeh, S. Olamafar Shepatoprotective effects of juglans regia extract against ccl4-induced oxidative damage in rats. Pharm. Biol. 2013, 51, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 11, 4092–4096. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds 1–17 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, B.; Liu, Z.; Jiang, Y.; Liu, Y.; Fu, L.; Wang, X.; Kuang, H. Cytotoxicity of Triterpenes from Green Walnut Husks of Juglans mandshurica Maxim in HepG-2 Cancer Cells. Molecules 2015, 20, 19252-19262. https://doi.org/10.3390/molecules201019252
Zhou Y, Yang B, Liu Z, Jiang Y, Liu Y, Fu L, Wang X, Kuang H. Cytotoxicity of Triterpenes from Green Walnut Husks of Juglans mandshurica Maxim in HepG-2 Cancer Cells. Molecules. 2015; 20(10):19252-19262. https://doi.org/10.3390/molecules201019252
Chicago/Turabian StyleZhou, Yuanyuan, Bingyou Yang, Zhaoxi Liu, Yanqiu Jiang, Yuxin Liu, Lei Fu, Xiaoli Wang, and Haixue Kuang. 2015. "Cytotoxicity of Triterpenes from Green Walnut Husks of Juglans mandshurica Maxim in HepG-2 Cancer Cells" Molecules 20, no. 10: 19252-19262. https://doi.org/10.3390/molecules201019252