Analysis of the Photophysical Behavior and Rotational-Relaxation Dynamics of Coumarin 6 in Nonionic Micellar Environments: The Effect of Temperature
Abstract
:1. Introduction

2. Results and Discussion
2.1. Spectroscopic Properties

| Medium | (λabs)max (nm) | (λem)max (nm) | Φf | τf a (ns) | kr (ns−1) | knr (ns−1) |
|---|---|---|---|---|---|---|
| EtOH | 457.0 | 501.0 | 0.78 | 2.57(1.08) | 0.304 | 0.085 |
| β-C12G2 | 465.0 | 505.5 | 0.77 | 3.21(1.07) | 0.240 | 0.072 |
| TX100 | 465.0 | 507.5 | 0.78 | 2.90(1.03) | 0.269 | 0.076 |
| C12E6 | 464.5 | 507.0 | 0.78 | 2.80(1.15) | 0.279 | 0.078 |

2.2. Temperature-Dependent Studies
2.2.1. Steady-State Fluorescence Anisotropy

2.2.2. Time-Resolved Fluorescence

2.2.3. Dynamic Light Scattering

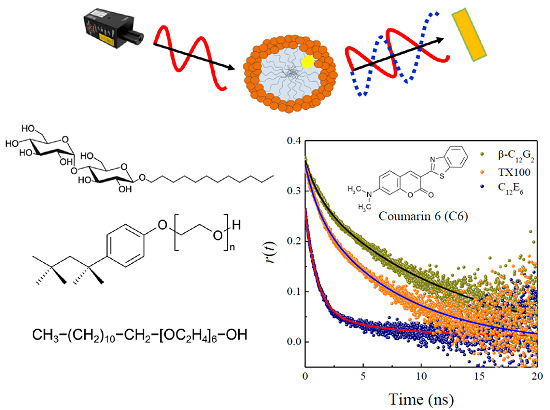
2.3. Time-Resolved Anisotropy Studies

| Micelles | T (K) | r0 | β a | τslow (ns) | τfast (ns) | χ2 | ‹τr› (ns) |
|---|---|---|---|---|---|---|---|
| 298.15 | 0.373 | 0.82 | 12.7 ± 0.8 | 1.4 ± 0.1 | 1.02 | 10.7 ± 3.0 | |
| β-C12G2 | 308.15 | 0.355 | 0.69 | 10.4 ± 1.0 | 1.8 ± 0.1 | 1.09 | 7.7 ± 1.8 |
| 318.15 | 0.359 | 0.45 | 13.0 ± 2.8 | 1.9 ± 0.1 | 1.10 | 6.9 ± 2.3 | |
| 298.15 | 0.361 | 0.76 | 8.1 ± 0.4 | 1.2 ± 0.1 | 1.01 | 6.4 ± 1.6 | |
| TX100 | 308.15 | 0.359 | 0.55 | 7.2 ± 0.8 | 1.4 ± 0.1 | 1.21 | 4.5 ± 1.4 |
| 318.15 | 0.338 | 0.25 | 7.7 ± 1.4 | 1.4 ± 0.1 | 1.18 | 3.0 ± 1.0 | |
| C12E6 | 298.15 | 0.306 | 0.16 | 10.8 ± 3.4 | 1.1 ± 0.1 | 1.21 | 2.7 ± 1.3 |
| Micelles | T (K) | ‹τr› (ns) | ‹τr› (ns) | τM (ns) | ηm (mPa·s) |
|---|---|---|---|---|---|
| β-C12G2 | 298.15 | 10.63 | 12.41 | 74.12 | 117.1 |
| 308.15 | 7.69 | 8.78 | 62.05 | 85.6 | |
| 318.15 | 6.88 | 8.03 | 48.09 | 80.8 | |
| TX100 | 298.15 | 6.43 | 6.86 | 102.80 | 64.7 |
| 308.15 | 4.53 | 4.65 | 170.59 | 45.4 | |
| 318.15 | 2.98 | 3.01 | 270.68 | 30.4 |
3. Experimental Section
3.1. Materials
| Chemical Name | Abbreviation | Source | Grade | Mass Fraction Purity |
|---|---|---|---|---|
| n-dodecyl-β-d-maltoside | β-C12G2 | Calbiochem | Ultrol | ≥0.99 |
| p-tert-octyl-Phenoxy polyethylene (9.5) ether | TX100 | Sigma-Aldrich | BioXtra | ≥0.98 |
| n-dodecyl-hexaoxyethylene-glycol | C12E6 | Sigma-Aldrich | BioXtra | ≥0.98 |
3.2. Methods
3.2.1. Steady-State Spectroscopic Measurements
3.2.2. Time-Resolved Measurements
3.2.3. Dynamic Light-Scattering Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; Wiley: Chichester, UK, 2003; pp. 1–37. [Google Scholar]
- Myers, D. Surfactant Science and Technology, 2nd ed.; VCH: New York, NY, USA, 1992; pp. 1–26. [Google Scholar]
- Petkov, J.T.; Tucker, I.M. The role of nanoscience in home and personal care products. In Nanoscience. Colloidal and Interfacial Aspects; Starov, V.M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1131–1145. [Google Scholar]
- Paul, B.K.; Gosh, N.; Mukherjee, S. Modulated photophysics and rotational-relaxation dynamics of coumarin 153 in nonionic micelles: The role of headgroup size and tail length of the surfactant. RSC Adv. 2015, 5, 9381–9388. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar] [PubMed]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Colloidal soft matters drug delivery system. J. Pharm. Sci. 2009, 98, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Soussan, E.; Cassel, S.; Blanzat, M.; Rico-Lattes, I. Drug delivery by soft matter: Matrix and vesicular carriers. Angew. Chem. Int. Ed. 2009, 48, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mall, S.; Buckton, G.; Rawlins, D.A. Dissolution behaviour of sulphonamides into sodium dodecyl sulfate micelles: A thermodynamic approach. J. Pharm. Sci. 1996, 85, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Volanschi, E. Spectral studies on the molecular interaction of anticancer drug mitoxantrone with CTAB micelles. J. Pharm. Sci. 2011, 100, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.K.; Ray, D.; Guchhait, N. Binding interaction and rotational-relaxation dynamics of a cancer cell photosensitizer with various micellar assemblies. J. Phys. Chem. B 2012, 116, 9704–9717. [Google Scholar] [CrossRef] [PubMed]
- Cesaretti, A.; Carlotti, B.; Gentili, P.L.; Clementi, C.; Germani, R.; Elisei, F. Spectroscopic investigation of the pH controlled inclusion of doxycycline and oxytetracycline antibiotics in cationic micelles and their magnesium driven release. J. Phys. Chem. B 2014, 118, 8601–8613. [Google Scholar] [CrossRef] [PubMed]
- Cesaretti, A.; Carlotti, B.; Consiglio, G.; Del Giacco, T.; Spalletti, A.; Elisei, F. Inclusion of two push-pull N-methylpyridinium salts in anionic surfactant solutions: A comprehensive photophysical investigation. J. Phys. Chem. B 2015, 119, 6658–6667. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Ghosh, S.; Banik, D.; Banerjee, C.; Kuchlyan, J.; Sarkar, N. An investigation into the effect of the structure of bile salt aggregates on the binding interactions and ESIHT dynamics of curcumin: A photophysical approach to probe bile salt aggregates as a potential drug carrier. J. Phys. Chem. B 2013, 117, 13795–13807. [Google Scholar] [CrossRef] [PubMed]
- Mcauley, W.J.; Jones, D.S.; Kett, V.L. Characterization of the interaction of lactate dehydrogenase with Tween-20 using isothermal titration calorimetry, interfacial rheometry and surface tension measurements. J. Pharm. Sci. 2009, 98, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.; Ribosa, I.; Campos, E.; Sánchez Leal, J. Ecological properties of alkylglucosides. Chemosphere 1997, 35, 545–556. [Google Scholar]
- Von Rybinski, W.; Hill, K. Alkyl Polyglycosides-properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Söderman, O.; Johansson, I. Polyhydroxyl-based surfactants and their physico-chemical properties and applications. Curr. Opin. Colloid Interface Sci. 2000, 4, 391–401. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Carnero Ruiz, C. Self-assembly and micellar structures of sugar-based surfactants: Effect of temperature and salt addition. In Sugar-Based Surfactants: Fundamentals and Applications; Carnero Ruiz, C., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 61–104. [Google Scholar]
- Garavito, R.M.; Ferguson-Miller, S. Detergents as tools in membrane biochemistry. J. Biol. Chem. 2001, 276, 32403–32406. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.; Linke, D. Phase separation in the isolation and purification of membrane proteins. Biotechniques 2007, 43, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Pillion, D.J.; Ahsan, F.; Arnold, J.J.; Balusubramanian, B.M.; Piraner, O.; Meezan, E. Synthetic long-chain alkyl maltosides and alkyl sucrose esters as enhancers of nasal insulin adsorption. J. Pharm. Sci. 2002, 91, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Kaatze, U. Ultrasonic spectrometry of aqueous solutions of alkyl maltosides: Kinetics of micelle formation and head-group isomerization. Chem. Phys. Chem. 2009, 10, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Carnero Ruiz, C.; Molina-Bolívar, J.M. Characterization of mixed non-ionic surfactants n-octyl-β-d-thioglucoside and octaethylene-glycol monododecyl ether: Micellization and microstructure. J. Colloid Interface Sci. 2011, 361, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Carnero Ruiz, C. Rotational dynamics of Coumarin 153 in non-ionic mixed micelles of n-octyl-β-d-thioglucoside and Triton X-100. Photochem. Photobiol. Sci. 2012, 11, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Molina-Bolívar, J.A.; Carnero Ruiz, C. Micellar size and phase behavior in n-octyl-β-d-thioglucoside/Triton X-100 mixtures: The effect of NaCl addition. Fluid Phase Equilib. 2012, 327, 58–64. [Google Scholar] [CrossRef]
- Hierrezuelo, J.M.; Carnero Ruiz, C. Rotational diffusion of coumarin 153 in nanoscopic micellar environments of n-dodecyl-β-d-maltoside and n-dodecyl-hexaethylene-glycol mixtures. J. Phys. Chem. A 2012, 116, 12476–12485. [Google Scholar] [CrossRef] [PubMed]
- Molina-Bolívar, J.A.; Hierrezuelo, J.M.; Carnero Ruiz, C. Energetics of clouding and size effects in non-ionic surfactant mixtures: The influence of alkyl chain length and NaCl addition. J. Chem. Thermodyn. 2013, 57, 59–66. [Google Scholar] [CrossRef]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Satpati, A.K.; Kumbhakar, M.; Maity, D.K.; Pal, H. Photophysical investigations of the solvent polarity effect on the properties of coumarin-6 dye. Chem. Phys. Lett. 2005, 407, 114–118. [Google Scholar] [CrossRef]
- Dutt, G.B.; Raman, S. Rotational dynamics of coumarins: And experimental test of dielectric friction theories. J. Chem. Phys. 2001, 114, 6702–6713. [Google Scholar] [CrossRef]
- Raikar, U.S.; Renuka, C.G.; Nadaf, Y.F.; Mulimani, B.G.; Karguppikar, A.M. Rotational diffusion and solvatochromic correlation of coumarin 6 laser dye. J. Fluoresc. 2006, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Dutt, G.B. Are the experimentally determined microviscosities of the micelles probe dependent? J. Phys. Chem. B 2004, 108, 3651–3657. [Google Scholar] [CrossRef]
- Finke, J.H.; Richter, C.; Gothsch, T.; Kwade, A.; Büttgenbach, S.; Müller-Goymann, C.C. Coumarin 6 as a fluorescent model drug: How to identify properties of lipid colloidal drug delivery systems via fluorescence spectroscopy? Eur. J. Lipid Sci. Technol. 2014, 116, 1234–1246. [Google Scholar] [CrossRef]
- Barooah, N.; Mohanty, J.; Pal, H.; Bhasikuttan, A.C. Stimulus-responsive supramolecular pKa tuning of cucurbit[7]uril encapsulated coumarin 6 dye. J. Phys. Chem. B 2012, 116, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Kumbhakar, M.; Mukherjee, T.; Pal, H. Temperature effect on the fluorescence anisotropy decay dynamics of coumarin-153 dye in Triton X-100 and Brij-35 micellar solutions. Photochem. Photobiol. 2005, 81, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Grieser, F.; Drummond, C.J. The physicochemical properties of self-assembled surfactant aggregates as determined by some molecular spectroscopic probe techniques. J. Phys. Chem. 1988, 92, 5580–5593. [Google Scholar] [CrossRef]
- Dutt, G.B. Rotational diffusion of hydrophobic probes in Brij-35 micelles: Effect of temperature on micellar internal environment. J. Phys. Chem. B 2003, 107, 10546–10551. [Google Scholar] [CrossRef]
- Matzinger, S.; Hussey, D.M.; Fayer, M.D. Fluorescence probe solubilization in the headgroup and core regions of micelles: Fluorescence lifetime and orientational relaxation measurements. J. Phys. Chem. B 1998, 102, 7216–7224. [Google Scholar] [CrossRef]
- Manna, A.; Chakravorti, S. Effect of micellar environment on charge transfer dye photophysics. J. Mol. Liq. 2012, 168, 94–101. [Google Scholar] [CrossRef]
- Inoue, T.; Misono, T. Cloud point phenomena for POE-type nonionic surfactants in a model room temperature ionic liquid. J. Colloid Interface Sci. 2008, 326, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kinosita, K.; Kawato, S.; Ikegami, A. Theory of fluorescence polarization decay in membranes. Biophys. J. 1977, 20, 289–305. [Google Scholar]
- Quitevis, E.L.; Marcus, A.H.; Fayer, M.D. Dynamics of ionic lipophilic probes in micelles: Picosecond fluorescence depolarization measurements. J. Phys. Chem. 1993, 97, 5762–5769. [Google Scholar] [CrossRef]
- Maiti, N.C.; Krishna, M.M.G.; Britto, P.J.; Periasamy, N. Fluorescence dynamics of dye probes in micelles. J. Phys. Chem. B 1997, 101, 11051–11060. [Google Scholar]
- Kapusta, P.; Erdmann, R.; Ortmann, U.; Wahl, M. Time-resolved fluorescence anisotropy measurements made simple. J. Fluoresc. 2003, 13, 179–183. [Google Scholar] [CrossRef]
- Choudhury, S.; Mondal, P.K.; Sharma, V.K.; Mitra, S.; Sakai, V.G.; Mukhopadhyay, R.; Pal, S.K. Direct observation of coupling between structural fluctuation and ultrafast hydration dynamics of fluorescent probes in anionic micelles. J. Phys. Chem. B 2015, 119, 10849–10857. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.A.; Drexhage, K.H. New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt. Commun. 1975, 13, 222–225. [Google Scholar]
- Candau, S.J. Light scattering. In Surfactant Solutions. New Methods of Investigation; Zana, R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1987; pp. 147–207. [Google Scholar]
- Provencher, S.W. CONTIN-a general-purpose constrained regularization program for inverting noisy linear algebraic and integral-equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, C.C.; Hierrezuelo, J.M.; Molina-Bolivar, J.A. Analysis of the Photophysical Behavior and Rotational-Relaxation Dynamics of Coumarin 6 in Nonionic Micellar Environments: The Effect of Temperature. Molecules 2015, 20, 19343-19360. https://doi.org/10.3390/molecules201019343
Ruiz CC, Hierrezuelo JM, Molina-Bolivar JA. Analysis of the Photophysical Behavior and Rotational-Relaxation Dynamics of Coumarin 6 in Nonionic Micellar Environments: The Effect of Temperature. Molecules. 2015; 20(10):19343-19360. https://doi.org/10.3390/molecules201019343
Chicago/Turabian StyleRuiz, Cristóbal Carnero, José Manuel Hierrezuelo, and José Antonio Molina-Bolivar. 2015. "Analysis of the Photophysical Behavior and Rotational-Relaxation Dynamics of Coumarin 6 in Nonionic Micellar Environments: The Effect of Temperature" Molecules 20, no. 10: 19343-19360. https://doi.org/10.3390/molecules201019343