Antihypertensive Effects of Artemisia scoparia Waldst in Spontaneously Hypertensive Rats and Identification of Angiotensin I Converting Enzyme Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preventive Effects of AS on Blood Pressure in SHR



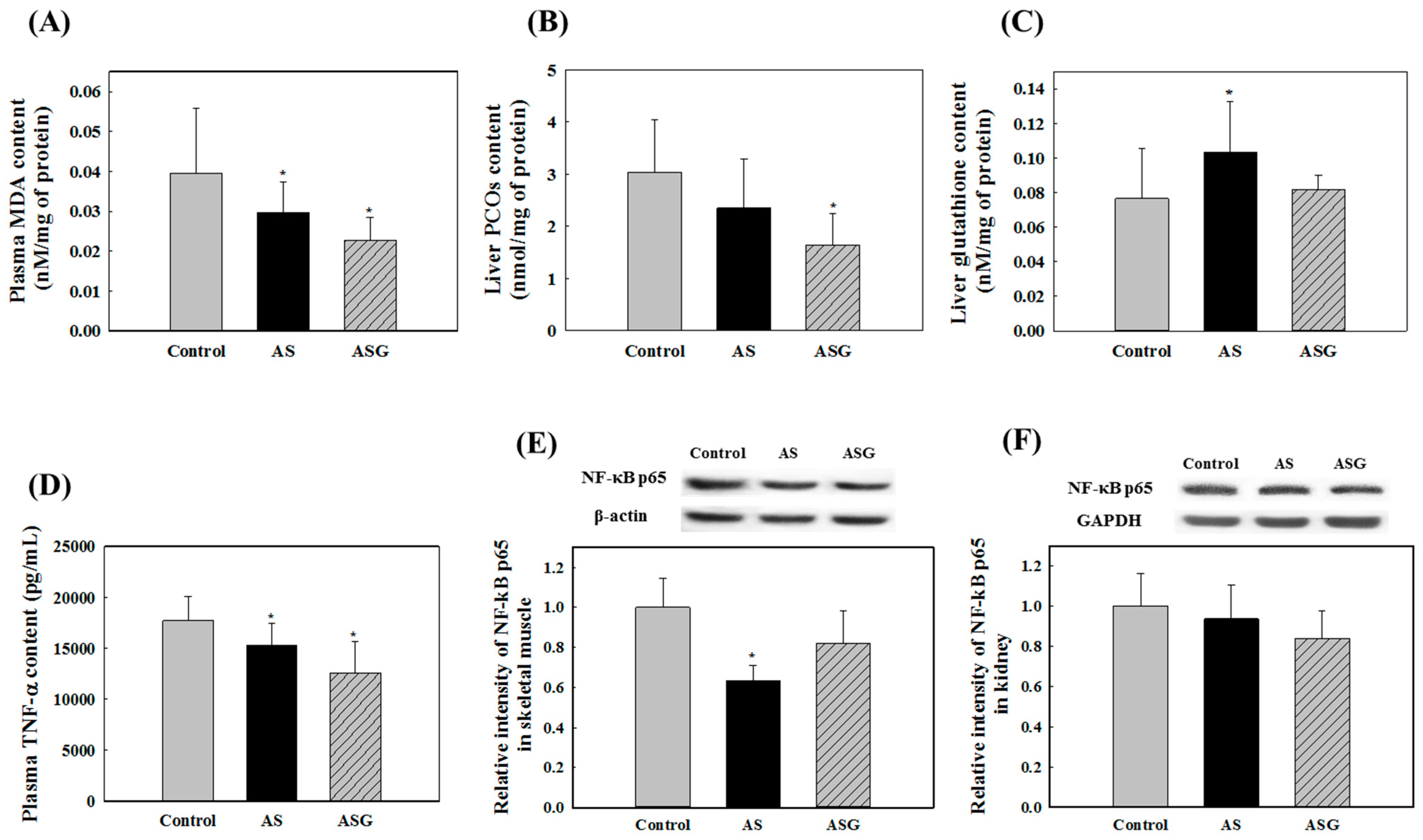
2.2. Preventive Effects of AS on Oxidative Stress in SHR

2.3. Isolation and Structural Determination of 2
| Position | 2 | |
|---|---|---|
| δH (rel. int., mult., J in Hz) | δC | |
| 1 | - | 131.7 |
| 2,6 | 8.01 (2H, dd, 8.4, 1.8) | 130.6 |
| 3,5 | 7.48 (2H, dd, 8.4, 8.4) | 129.7 |
| 4 | 7.60 (1H, tt, 8.4, 1.8) | 134.4 |
| 7 | - | 168.1 |
| 1′a | 4.31 (1H, d, 12.6) | 62.5 |
| 1′b | 4.11 (1H, d, 12.6) | |
| 2′ | 5.83 (1H, m) | 122.3 |
| 3′ | - | 139.6 |
| 4′ | 4.92 (2H, s) (a) | 74.6 |
| 5′ | 1.84 (3H, s) | 14.5 |
| 1′′ | 4.28 (1H, d, 7.8) | 103.3 |
| 2′′ | 3.21 (1H, dd, 9.0, 7.8) | 75.2 |
| 3′′ | 3.34 (1H, t, 9.0) (b) | 78.2 |
| 4′′ | 3.28 (1H, t, 9.0) | 71.8 |
| 5′′ | 3.24 (1H, m) | 78.2 |
| 6′′a | 3.65 (1H, dd, 12.0, 5.4) | 62.9 |
| 6′′b | 3.86 (1H, dd, 12.0, 2.4) | |
2.4. ACE Inhibitory Activity of the Isolated Compounds

3. Experimental Section
3.1. General Experimental Procedures
3.2. Chemicals
3.3. Materials
3.4. Animal Studies
3.5. Blood Pressure Measurement
3.6. Determination of Plasma ACE Activity
3.7. Determination of Serum Angiotensin II content
3.8. Determination of NO Content in Plasma
3.9. Western Blotting
3.10. Determination of Thiobarbituric Acid Reactive Substances (TBARS)
3.11. Determination of Protein Carbonyl Content
3.12. Measurement of Glutathione (GSH) Content
3.13. Determination of TNF-α Level in Plasma
3.14. Purification and Isolation of ACE Inhibitor in AS Water Extract
3.15. 4′-O-β-d-Glucopyranoyl (E)-4-hydroxy-3-methylbut-2-enyl benzoate (2)
3.16. Assay for ACE Inhibitory Activity
3.17. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drazner, M.H. The progression of hypertensive heart disease. Circulation 2011, 123, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Player, M.S.; Peterson, L.E. Anxiety disorders, hypertension, and cardiovascular risk: A review. Int. J. Psychiatry Med. 2011, 41, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonner, E.; Muller, M.; Oberritter, H.; Achulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am. J. Hypertens. 2012, 25, 180–189. [Google Scholar] [CrossRef] [PubMed]
- McCall, D.O.; McGartland, C.P.; McKinley, M.C.; Patterson, C.C.; Sharpe, P.; McCance, D.R.; Young, I.S.; Woodsice, J.V. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation 2009, 119, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.H.; Park, H.J.; Cho, J.Y. Salicornia herbacea: Botanical, chemical and pharmacological review of halophyte marsh plant. J. Med. Plants Res. 2009, 3, 548–555. [Google Scholar]
- Priyashree, S.; Pattanayak, S.P. A review on Cressa cretica Linn.: A halophytic plant. Pharmacogn. Rev. 2010, 4, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legualt, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Joseph, D.; Chakraborty, K.; Subin, C.S.; Vijayan, K.K. Halophytes of Chenopodiaceae and Aizoaceae from south-east coast of India as potential sources of essential nutrients and antioxidants. J. Food Nutr. Res. 2013, 1, 97–107. [Google Scholar]
- Yang, C.; Zheng, S.; Huang, H.; Liu, Z.; Zheng, W.; Liu, B.; Shi, D. Comparison of osmatic adjustment and ion balance strategies in nineteen alkali-tolerant halophyte species during adaptation to salt-alkalinized habitats in northeast China. Aust. J. Crop Sci. 2012, 6, 141–148. [Google Scholar]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Park, K.Y.; Lee, S.H.; Ham, K.S.; Lee, H.J.; Park, K.H.; Moon, J.H. Dicaffeoylquinic acid derivatives and flavonoid glucosides from glasswort (Salicornia herbacea L.) and their antioxidative activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Yang, X.; Lily, J.; Feng, L.; Ham, K.S. Screening on chemical constituent and biological activities of various halophytes. Food Sci. Biotechnol. 2015. to be submitted. [Google Scholar]
- Singh, H.P.; Kaur, S.; Mittal, S.; Batish, D.R.; Kohli, R.K. In vitro screening of essential oil from young and mature leaves of Artemisia scoparia compared to its major constituents for free radical scavenging activity. Food Chem. Toxicol. 2010, 48, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kim, J.Y.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta Med. 2005, 71, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Hsiao, G.; Chang, J.W.; Sheu, J.R.; Yen, M.H. Scoparone inhibits tissue factor expression in lipopolysaccharide-activated human umbilical vein endothelial cells. Biomed. Sci. 2003, 10, 518–525. [Google Scholar] [CrossRef]
- Pan, S.L.; Huang, Y.W.; Guh, J.H.; Chang, Y.L.; Peng, C.Y.; Teng, C.M. Esculetin inhibits ras-mediated cell proliferation and attenuates vascular restenosis following angioplasty in rats. Biochem. Pharmacol. 2003, 65, 1897–1905. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, H.C.; Weng, Y.I.; Yu, Y.J.; Lee, Y.T. Morphological evidence for the antiatherogenic effect of scoparone in hyperlipidemic diabetic rabbit. Cardiavasc. Res. 1994, 28, 1679–1685. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, X.H.; Yu, Y.; Tipton, R.C.; Raskin, I.; Ribnicky, D.; Johnson, W.; Cefalu, W.T. Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism 2013, 62, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, T.; Yakura, N.; Matsuzaki, K.; Kitanaka, S. Inhibitory effect of chemical constituents from Artemisia scoparia Waldst. et Kit. on triglyceride accumulation in 3T3-L1 cells and nitric oxide production in Raw 264.7 cells. J. Nat. Med. 2014, 68, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecień, I.; Wlodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Shouk, R.; Abdou, A.; Shetty, K.; Sarkar, D.; Eid, A.H. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr. Res. 2014, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 414–420. [Google Scholar]
- Ku, D.D.; Abdel-Razek, T.T.; Dai, J.; Kim-Park, S.; Fallon, M.B.; Abrams, G.A. Garlic and its active metabolite allicin produce endothelium- and nitric oxide-dependent relaxation in rat pulmonary arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.; Squadrito, G.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Doeller, J.E.; Darley-Usmar, V.M.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2013, 204, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P.; Sullivan, T. Effect of garlic on blood pressure: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.A.; Silva, M.E. Animal models for the study of arterial hypertension. J. Biosci. 2011, 36, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.S.; Kim, S.W.; Kim, C.J. Overview of the renin-angiotensin system. Korean Circ. J. 2007, 37, 91–96. [Google Scholar] [CrossRef]
- Sridevi, P.; Prashanth, K.S.; Bhagavan Raju, M. Angiotensin converting enzyme: A target for anti-hypertensive drugs. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 63–72. [Google Scholar]
- Kim, S.; Iwao, H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2011, 52, 12–30. [Google Scholar]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Flammer, A.; Lüuscher, T.F. Nitric oxide in hypertension. J. Clin. Hypertens. 2006, 8 (Suppl. 4), 17–29. [Google Scholar] [CrossRef]
- Facemire, C.S.; Nixon, A.B.; Griffiths, R.; Hurwits, H.; Coffman, T.M. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009, 54, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Bei, Y.; Liu, Z.; Dinh-Xuan, A.T. Role of Rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol. Ther. 2013, 137, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, C.; Lasagni, L.; Romagnai, P.; Bellini, F.; Mannelli, M.; Misciglia, N.; Mavila, C.; Vellei, U.; Villari, D.; Serio, M. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J. Am. Soc. Nephrol. 1999, 10, 245–255. [Google Scholar] [PubMed]
- Bregeon, J.; Loirand, G.; Pacaud, P.; Rolli-Derkinderen, M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2009, 297, C1062–C1070. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.J.; Corbertt, L.; Stocks, J.; Holiand, E.; Zhu, Y.M.; Pang, L. Human airway smooth muscle cells secrete vascular endothelial growth factor: Up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J. 2001, 15, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.C.; Pellegrin, M.; Wyss, C.; Abert, J.F.; Nussberger, J.; Hayoz, D.; Lehr, H.A; Mazzolai, L. Intravital microscopy reveals endothelial dysfunction in resistance arterioles in angiotensin II-induced hypertension. Hypertens. Res. 2012, 35, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F. Lipid oxidation and peroxidation in CNS health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 125–169. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Menini, S.; Maineri, E.P.; Patriarca, S.; Odetti, P.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A. Malondialdehyde, a lipoperoxidation-derived aldehyde, can bring about secondary oxidative damage to proteins. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 890–895. [Google Scholar] [CrossRef]
- Vaziri, N.D. Causal link between oxidative stress, inflammation, and hypertension. Iran J. Kidney Dis. 2008, 2, 1–10. [Google Scholar] [PubMed]
- Ide, N.; Lau, B.H. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-κB activation. J. Nutr. 2001, 131, 1020S–1026S. [Google Scholar] [PubMed]
- Fu, M.; Zhou, J.; Sun, A.; Zhang, S.; Zhang, C.; Zou, Y.; Fu, M.; Ge, J. Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction—A meta analysis of 7 prospective clinical studies. Int. J. Cardiol. 2012, 155, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zuanetti, G.; Latini, R.; Maggioni, P.; Franzosi, M.; Santoro, L.; Tognoni, G. Effect of the ACE inhibitor lisinopril on mortality in diabetic patients with acute myocardial infraction. Circulation 1997, 96, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Amann, B.; Tinzmann, R.; Angelkort, B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care 2003, 26, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Giner, R.M.M.; Máñez, S.; Ríos, J.L. New acetophenone glucosides isolated from extracts of Helichrysum italicum with antiinflammatory activity. J. Nat. Prod. 2001, 64, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Banday, J.A.; Mir, F.A.; Farooq, S.; Qurishi, M.A.; Koul, S.; Razdan, T.K. Salicylic acid and methyl gallate from the roots of Conyza canedensis. Int. J. Chem. Anal. Sci. 2012, 3, 1305–1308. [Google Scholar]
- Chung, J.H.; Shin, H.C.; Cho, J.Y.; Kang, S.K.; Lee, H.J.; Shin, S.C.; Park, K.H.; Moon, J.H. Isolation and structural determination of free radical scavenging compounds from Korean fermented red pepper past (kochujang). Food Sci. Biotechnol. 2009, 18, 463–470. [Google Scholar]
- Wang, X.Y.; Xu, M.; Yang, C.R.; Zhang, Y.J. Phenylpropanoid glycosides from the seeds of Michelia hedyosperma. Food Chem. 2011, 126, 1039–1043. [Google Scholar] [CrossRef]
- Simaratanamongkol, A.; Umehara, K.; Noguchi, H.; Panichayupakaranant, P. Identification of a new angiotensin-converting enzyme (ACE) inhibitor from Thai edible plants. Food Chem. 2014, 165, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, B.W.N.; Rupasinghe, H.P.V. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct. Foods Health Dis. 2011, 5, 172–188. [Google Scholar]
- Actis-Goretta, L.; Ottaviani, J.I.; Fraga, C.G. Inhibition of angiotensin converting enzyme activity by flavonol-rich foods. J. Agric. Food Chem. 2006, 11, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 11, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Gonzáleza, R.; Ballesterc, I.; López-Posadasa, R.; Suárezb, M.D.; Martínez-Augustinb, O.; Zarzuelob, A.; Sánchez de Medinaa, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Moshage, H.; Kok, B.; Huizenga, J.R.; Jansen, P.L.M. Nitrite and Nitrate determinations in plasma: A critical evaluation. Chin. Chem. 1995, 41, 892–896. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ledwozyw, A.; Michalak, J.; Stepian, A.; Kadziolka, A. The relationship between plasma TG, cholesterol, total lipid peroxidation product during human atherosclerosis. Clin. Chim. Acta 1986, 155, 272–284. [Google Scholar]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Meth. Enzymol. 1994, 233, 357–363. [Google Scholar] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-Y.; Park, K.-H.; Hwang, D.Y.; Chanmuang, S.; Jaiswal, L.; Park, Y.-K.; Park, S.-Y.; Kim, S.-Y.; Kim, H.-R.; Moon, J.-H.; et al. Antihypertensive Effects of Artemisia scoparia Waldst in Spontaneously Hypertensive Rats and Identification of Angiotensin I Converting Enzyme Inhibitors. Molecules 2015, 20, 19789-19804. https://doi.org/10.3390/molecules201119657
Cho J-Y, Park K-H, Hwang DY, Chanmuang S, Jaiswal L, Park Y-K, Park S-Y, Kim S-Y, Kim H-R, Moon J-H, et al. Antihypertensive Effects of Artemisia scoparia Waldst in Spontaneously Hypertensive Rats and Identification of Angiotensin I Converting Enzyme Inhibitors. Molecules. 2015; 20(11):19789-19804. https://doi.org/10.3390/molecules201119657
Chicago/Turabian StyleCho, Jeong-Yong, Kyung-Hee Park, Do Young Hwang, Saoraya Chanmuang, Lily Jaiswal, Yang-Kyun Park, Sun-Young Park, So-Young Kim, Haeng-Ran Kim, Jae-Hak Moon, and et al. 2015. "Antihypertensive Effects of Artemisia scoparia Waldst in Spontaneously Hypertensive Rats and Identification of Angiotensin I Converting Enzyme Inhibitors" Molecules 20, no. 11: 19789-19804. https://doi.org/10.3390/molecules201119657





