Optical and Electrical Properties of TTF-MPcs (M = Cu, Zn) Interfaces for Optoelectronic Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
| Sample | ν(C-C) Isoindole Deformation | ν(C-N) Stretch in Pyrrole Ring | ν(C-N) in Plane Isoindole and Stretching Vibration | ν(C-H) in Plane Bending | Benzene Ring | TTF |
|---|---|---|---|---|---|---|
| CuPc-TTF Pellet | 1611 | 1065 | 1288, 761 | 1421 | 697 | 1508, 715, 694 |
| CuPc-TTF Thin Film | 1596 | 1079 | 1287, 751 | 1437 | 697 | 1508, 721 |
| CuPc-TTF (annealing 353 K) | 1605 | 1080 | 1283, 774 | 1438 | 1508, 726 | |
| CuPc-TTF (annealing 393 K) | 1596 | 1079 | 1287, 751 | 1437 | 697 | 1507, 721 |
| ZnPc-TTF Pellet | 1606 | 1075 | 1278, 750 | 1409 | 695 | 1500, 778, 683 |
| ZnPc-TTF Thin Film | 1596 | 1108 | 1284, 750 | 1420 | 1508, 778 | |
| ZnPc-TTF (annealing 353 K) | 1609 | 1111 | 1284, 748 | 1422 | 1508, 779 | |
| ZnPc-TTF (annealing 393 K) | 1609 | 1110 | 1284, 749 | 1424 | 1508, 774 |

2.2. Microscopy Study

| Thin Film | Film Thickness (Å) | RMS Roughness (nm) | Indirect Fundamental Energy Gap, Eg1 (eV) | Eg2 and Eg3 (eV) |
|---|---|---|---|---|
| TTFderiv-CuPc | 11,667 | 1700 | 1.4 | 1.5, 2.8 |
| TTFderiv-ZnPc | 1620 | 66 | 1.7 | 1.3, 2.0 |

2.3. Optical Properties

2.4. Electrical Properties




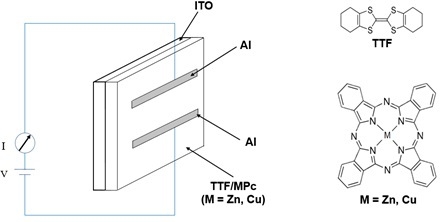
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Logothetidis, S. Flexible organic electronic devices: Materials, process and applications. Mater. Sci. Eng. B 2008, 152, 96–104. [Google Scholar] [CrossRef]
- Rand, B.P.; Cheyns, D.; Vasseur, K.; Giebink, N.C.; Mothy, S.; Yi, Y.; Coropceanu, V.; Beljonne, D.; Cornil, J.; Brédas, J.L.; et al. The impact of molecular orientation on the photovoltaic properties of a phthalocyanine/fullerene heterojunction. Adv. Funct. Mater. 2012, 22, 2987–2995. [Google Scholar] [CrossRef]
- Rajesh, K.R.; Menon, C.S. D.C. electrical and optical properties of vacuum-deposited organic semiconductor FePcCl thin films. Can. J. Phys. 2005, 83, 1151–1159. [Google Scholar] [CrossRef]
- Shafai, T.S.; Anthopoulos, T.D. Junction properties of nickel phthalocyanine thin film devices utilizing indium injecting electrodes. Thin Solid Films 2001, 398–399, 361–367. [Google Scholar] [CrossRef]
- Gravano, S.; Hassan, A.K.; Gould, R.D. Effects of annealing on the trap distribution of cobalt phthalocyanine thin films. Int. J. Electron. 1991, 70, 477–484. [Google Scholar] [CrossRef]
- Hassan, A.K.; Gould, R.D. The interpretation of current density-voltage and activation energy measurements on freshly prepared and heat treated nickel phthalocyanine thin films. In. J. Electron. 1993, 74, 59–65. [Google Scholar] [CrossRef]
- Peltekis, N.; Holland, B.N.; Krishnamurthy, S.; McGovern, I.T.; Poolton, N.R.J.; Patel, S.; McGuinness, C. Electronic and optical properties of magnesium phthalocyanine (MgPc) solid films studied by soft X-ray excited optical luminescence and X-ray absorption spectroscopies. J. Am. Chem. Soc. 2008, 130, 13008–13012. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Li, W.L.; Chu, B.; Lee, C.S.; Su, Z.S.; Zhang, G.; Wu, S.H.; Yan, F. High speed responsive near infrared photodetector focusing on 808 nm radiation using hexadecafluoro-copper-phthalocyanine as the acceptor. Org. Electron. 2011, 12, 34–38. [Google Scholar] [CrossRef]
- Bialek, B.; Kim, I.G.; Lee, J.I. Ab initio study of the electronic structure of nickel phthalocyanine-monolayer and bulk. Synth. Met. 2002, 129, 151–156. [Google Scholar] [CrossRef]
- Bendikov, M.; Wudl, F.; Perepichka, D.F. Tetrathiafulvalenes, oligoacenenes, and their buckministerfullerene derivatives: The brick and mortar of organic electronics. Chem. Rev. 2004, 104, 4891–4945. [Google Scholar] [CrossRef] [PubMed]
- Farren, C.; Christensen, C.A.; FitzGerald, S.; Bryce, M.R.; Beeby, A. Synthesis of novel Phthalocyanine-Tetrathiafulvalene hybrids; intramolecular fluorescence quenching related to molecular geometry. J. Org. Chem. 2002, 67, 9130–9139. [Google Scholar] [CrossRef]
- Molas, S.; Caro, J.; Santiso, J.; Figueras, A.; Fraxedas, J.; Méziére, C.; Fourmigué, M.; Batail, P. Thin molecular films of neutral tetrathiafulvalene-derivatives. J. Cryst. Growth 2000, 218, 399–409. [Google Scholar] [CrossRef]
- Wang, C.; Bryce, M.R.; Batsanov, A.S.; Stanley, C.F.; Beeby, A.; Howard, J.A.K. Synthesis, spectroscopy and electrochemistry of phthalocyanine derivatives functionalized with four and eight peripheral tetrathiafulvalene units. J. Chem. Soc. Pekin Trans. 1997, 2, 1671–1678. [Google Scholar] [CrossRef]
- Cook, M.J.; Cooke, G.; Jafari-Fini, A. A liquid crystalline tetrathiafulvalenylphthalocyanine. Chem. Commun. 1996, 1925–1926. [Google Scholar] [CrossRef]
- Blower, M.A.; Bryce, M.R.; Devonport, W. Synthesis and aggregation of a phthalocyanine symmetrically-functionalized with eight tetrathiafulvalene units. Adv. Mater. 1996, 8, 63–65. [Google Scholar] [CrossRef]
- Wang, C.; Bryce, M.R.; Batsanov, A.S.; Howard, J.A.K. Synthesis of pyrazinoporphyrazine derivatives functionalised with tetrathiafulvalene (ttf) units: X-ray crystal structures of two related ttf cyclophanes and two bis(1,3-dithiole-2-thione) intermediates. Chem. Eur. J. 1997, 3, 1679–1690. [Google Scholar] [CrossRef]
- Nitschke, C.; O’Flaherty, S.M.; Kroell, M.; Strevens, A.; Maier, S.; Ruether, M.G.; Blau, W.J. Preparation and nonlinear optical properties of phthalocyanine nanocrystals. In Organic Photonic Materials and Devices V, Proceedings of the SPIE, San Jose, CA, USA, 25 January 2003; Grote, J.G., Kaino, T., Eds.; Volume 4991, pp. 124–132.
- Mahapatro, A.K.; Ghosh, S. Charge carrier transport in metal phthalocyanine based disordered thin films. J. Appl. Phys. 2007, 101, 034318. [Google Scholar] [CrossRef]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.J. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Rivera, M. Investigation of optical properties of annealed aluminum phthalocyanine derivatives thin films. J. Phys. Chem. Solids 2014, 75, 599–605. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Solimana, H.S.; Khalifab, B.A.; Soliman, I.M. Structural and optical properties of nanocrystalline aluminum phthalocyanine chloride thin films. Mater. Sci. Semicond. Process. 2015, 38, 177–183. [Google Scholar] [CrossRef]
- Melby, L.R.; Hartzler, H.D.; Sheppard, W.A. An improved synthesis of tetrathiafulvalene. J. Org. Chem. 1974, 39, 2456–2458. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Al-Ghamdi, A.A.; Asiri, A.M. Optical properties of thermally evaporated tin-phthalocyanine dichloride thin films, SnPcCl2. Phys. B Condens. Matter 2004, 334, 398–406. [Google Scholar] [CrossRef]
- Robinet, S.; Clarisse, C.; Gauneau, M.; Salvi, M. Spectroscopic and structural studies of scandium diphthalocyanine films. Thin Solid Films 1989, 182, 307–317. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Darwish, A.A.A. Dispersion studies and electronic transitions in nickel phthalocyanine thin films. Opt. Laser Technol. 2005, 37, 513–523. [Google Scholar] [CrossRef]
- Noguchi, T.; Gotoh, K.; Yamaguchi, Y. Novel method to disperse ultrafine metal particles into polymer. J. Mater. Sci. Lett. 1991, 10, 477–479. [Google Scholar] [CrossRef]
- Neghabi, M.; Zadsar, M.; Bagher Ghorashi, S.M. Investigation of structural and optoelectronic properties of annealed nickel phthalocyanine thin films. Mater. Sci. Semicond. Process. 2014, 17, 13–20. [Google Scholar] [CrossRef]
- Laidani, N.; Bartali, R.; Gottardi, G.; Anderle, M.; Cheyssac, P. Optical absorption parameters of amorphous carbon films from Forouhi-Bloomer and Tauc-Lorentz models: A comparative study. J. Phys. Condens. Matter 2008, 20, 015216. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Salam, M.M.; Ali, H.A. Optical properties of thermally evaporated metal-free phthalocyanine (H2Pc) thin films. Int. J. Mod. Phys. B 2005, 19, 4057–4071. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Darwish, A.A.A. Fourier-transform infrared and UV-vis spectroscopes of nickel phthalocyanine thin films. Mater. Chem. Phys. 2005, 92, 185–189. [Google Scholar] [CrossRef]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. Ultraviolet and visible spectroscopic studies of phthalocyanine and its complexes thin films. Opt. Mater. 2006, 29, 304–312. [Google Scholar] [CrossRef]
- O’Leary, S.K.; Lim, P.K. On determining the optical gap associated with an amorphous semiconductor: A generalization of the Tauc model. Solid State Commun. 1997, 104, 17–21. [Google Scholar] [CrossRef]
- Mok, T.M.; O’Leary, S.K. The dependence of the Tauc and Cody optical gaps associated with hydrogenated amorphous silicon on the film thickness: αl experimental limitations and the impact of curvature in the Tauc and Cody plots. J. Appl. Phys. 2007, 102, 113525. [Google Scholar] [CrossRef]
- Leontie, L.; Roman, M.; Brinza, F.; Podaru, C.; Rusu, G.I. Electrical and optical properties of some new synthesized ylides in thin films. Synth. Met. 2002, 138, 157–163. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, A.; Sánchez-Hernández, C.M.; Fleitman-Levin, I.; Arenas-Alatorre, J.; Alonso-Huitrón, J.C.; Sánchez Vergara, M.E. Optical absorption and visible photoluminescence from thin films of silicon phthalocyanine derivatives. Materials 2014, 7, 6585–6603. [Google Scholar] [CrossRef]
- Adachi, S. Optical Properties of Crystalline and Amorphous Semiconductors: Materials and Fundamental Principle; Springer Science & Business Media: New York, NY, USA, 1999. [Google Scholar]
- Anthopoulos, T.D.; Shafai, T.S. SCLC measurements in nickel phthalocyanine thin films. Phys. Status Solidi (a) 2000, 181, 569–574. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds TTFderivative-MPc (M = Cu, Zn) are not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Vergara, M.E.; Leyva-Esqueda, M.; Alvárez-Bada, J.R.; García-Montalvo, V.; Rojas-Montoya, I.D.; Jiménez-Sandoval, O. Optical and Electrical Properties of TTF-MPcs (M = Cu, Zn) Interfaces for Optoelectronic Applications. Molecules 2015, 20, 21037-21049. https://doi.org/10.3390/molecules201219742
Sánchez-Vergara ME, Leyva-Esqueda M, Alvárez-Bada JR, García-Montalvo V, Rojas-Montoya ID, Jiménez-Sandoval O. Optical and Electrical Properties of TTF-MPcs (M = Cu, Zn) Interfaces for Optoelectronic Applications. Molecules. 2015; 20(12):21037-21049. https://doi.org/10.3390/molecules201219742
Chicago/Turabian StyleSánchez-Vergara, María Elena, Mariel Leyva-Esqueda, José Ramón Alvárez-Bada, Verónica García-Montalvo, Iván Darío Rojas-Montoya, and Omar Jiménez-Sandoval. 2015. "Optical and Electrical Properties of TTF-MPcs (M = Cu, Zn) Interfaces for Optoelectronic Applications" Molecules 20, no. 12: 21037-21049. https://doi.org/10.3390/molecules201219742