Antinociceptive Activity and Toxicity Evaluation of the Fatty Oil from Plukenetia polyadenia Mull. Arg. (Euphorbiaceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oil-Composition
| Constituents | RICalc | RILit | Oil % | Identification |
|---|---|---|---|---|
| Palmitic acid, methyl ester | 1925 | 1921 | 2.9 | GC, MS |
| Linoleic acid, methyl ester | 2097 | 2092 | 46.5 | GC, MS |
| α-Linolenic acid, methyl ester | 2104 | 2098 | 34.4 | GC, MS |
| Oleic acid, methyl ester | 2112 | 2107 | 13.9 | GC, MS |
| Stearic acid, methyl ester | 2129 | 2124 | 1.5 | GC, MS |
| Arachidonic acid, methyl ester | 2279 | 2274 | 0.8 | GC, MS |
| Total | 100.0 | GC, MS |
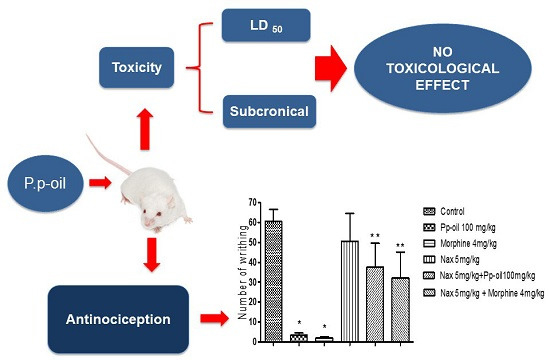
2.2. Acute Toxicity (LD50)
2.3. Clinical Chemistry and Histophatology



2.4. Acetic Acid-Induced Writhing

2.5. Hot Plate Test

2.6. Formalin Test

2.7. Mechanism of Action

2.8. Discussion
3. Experimental Section
3.1. Drugs and Chemicals
3.2. Animals
3.3. Plant Material
3.4. Fatty Acids Esterification
3.5. Oil-Composition Analysis
3.6. NMR Analysis of Pp-Oil
3.7. Acute Toxicity (LD50)
3.8. 30-Day Chronic Oral Toxicity (Experimental Design)
3.9. Histopathology
3.10. Clinical Tests
3.11. Antinociceptive Activity
3.11.1. Acetic Acid-Induced Writhing in Mice
3.11.2. Hot Plate Test
3.11.3. Formalin Test
3.11.4. Evaluation of the Mechanism of Action
3.12. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Plant List. Available online: http://www.theplantlist.org/tpl/record/kew-161498 (accessed on 27 November 2014).
- Gillespie, L.J.; Armbruster, W.S. A Contribution to the Guianan Flora: Dalechampia,Haematostemon,Omphalea,Pera,Plukenetia and Tragia (Euphorbiaceae) with Notes on Subfamily Acalyphoideae; Smithsonian Institution Press: Washington, DC, USA, 1997. [Google Scholar]
- Ribeiro, A.F. Chemical and Toxicological Evaluation of the Fatty Oil from Plukenetia polyadenia Müll. Arg. (Euphorbiaceae). Master’s Degree Dissertation, Graduate Program in Chemistry, Universidade Federal do Pará, Belém, PA, Brazil, 15 03 2005. [Google Scholar]
- Grimble, R.F.; Tappia, P.S. Modulation of pro-inflammatory cytokine biology by unsaturated fatty acids. Z. Ernahr. 1998, 37, 57–65. [Google Scholar]
- O’Shea, M.; Bassaganya-Riera, J.; Mohede, I.C. Immunomodulatory properties of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1199–1206. [Google Scholar]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D.; Shuka, V.K.S. NMR of lipids. Ann. Rep. NMR Spectrosc. 1995, 31, 219–237. [Google Scholar]
- Knothe, G.; Kenar, J.A. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- NIST (National Institute of Standards and Technology). Mass Spectral Library (NIST/EPA/NIH, v. 2.0d); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Adams, R.P. Identification of Essencial Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol stream, IL, USA, 2007. [Google Scholar]
- Ramos, L.C.S.; Tango, J.S.; Savi, A.; Leal, N.R. Variability for oil and fatty acid composition in castor bean varieties. J. Am. Oil Chem. Soc. 1984, 61, 1841–1843. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K.; Sporer , F.; Wink, M. Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. J. Agric. Food Chem. 1997, 45, 3152–3157. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccione, E.; Cittadini, A.R.M.; Calviello, G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, G.V.; Broadwin, R.; Liaw, J.; Dawson, S.V. Characterization of the LOAEL-to-NOAEL uncertainty factor for mild adverse effects from acute inhalation exposures. Regul. Toxicol. Pharm. 2002, 36, 96–105. [Google Scholar] [CrossRef]
- GHS—The Globally Harmonized System of classification and labelling of chemicals. Health and Environmental Hazards Classification Criteria for Substances; United Nations Economics Commission for Europe, 2007, 2nd ed. Available online: http://www.unece.org/trans/danger/publi/ghs/GHS_presentations/English/health_env_sub_e.pdf (accessed on 11 December 2014).
- Tjølsen, A.; Hole, K. Animal Models of Analgesia. In The Pharmacology of Pain; Dickenson, A., Besson, J., Eds.; Springer Verlag: Berlin, Germany, 1997; Volume 130, pp. 1–20. [Google Scholar]
- Correa, C.R.; Kyke, D.J.; Chakravert, S.; Calixto, J.B. Antinociceptive profile of the pseudopeptide B2 bradykinin and receptor antagonist NPC 18688 in mice. Br. J. Pharmacol. 1996, 117, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ugwah-Oguejiofor, C.J.; Abubakar, K.; Ugwah, M.O.; Njan, A.A. Evaluation of the antinociceptive and anti-inflammatory effect of Caralluma dalzielii. J. Ethnopharmacol. 2013, 150, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Khalfoun, B.; Thibault, F.; Watier, H.; Bardos, P.; Lebranchu, Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production interleukin-6. Adv. Exp. Med. Biol. 1997, 400, 589–597. [Google Scholar]
- Calder, P.C. Mechanism of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Ghani, Z.D.F.A.; Nor, R.N.S.R.M.; Gopalan, H.K.; Sulaiman, M.R.; Jais, A.M.M.; Somchit, M.N.; Kader, A.A.; Ripin, J. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J. Nat. Med. 2008, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, Y.; Guo, S.; Shen, Z.; Wang, Y.; Yang, L. Anti-inflammatory and analgesic activity of aqueous extract of Flos populi. J. Ethnopharmacol. 2014, 152, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Aloisi, A.M. Refinement of pain evaluation techniques: The formalin test. Ann. Ist. Super. Sanita 2004, 40, 223–229. [Google Scholar] [PubMed]
- Amaral, J.F.; Silva, M.I.G.; Aquino Neto, M.R.; Teixeira Neto, P.F.; Moura, B.A.; de Melo, C.T.V.; de Araújo, F.L.O.; de Sousa, D.P.; de Vasconcelos, P.F.; de Vasconcelos, S.M.M.; et al. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 2007, 30, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.R.; Oliveira, F.S.; Benedito, R.B.; Sousa, D.P.; Almeida, R.N.; Araújo, D.A.M.A.D. Antinociceptive activity of (−)-carvone: Evidence of association with decreased peripheral nerve excitability. Biol. Pharm. Bull. 2008, 31, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Kanna, N.; Bathia, J. Antinociception action of Ocimum sanctum (Tulsi) in mice: Possible mechanisms involved. J. Ethnopharmacol. 2003, 88, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; Sousa, D.P.; Almeida, R.N. Antinociceptive effect of hydroxydihydrocarvone. Biol. Pharm. Bull. 2008, 31, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, P.; Tang, C.; Wang, Y.; Li, Y.; Zhang, H. Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol. 2014, 151, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Kaithwas, G. Anti-inflammatory potential of alpha-linolenic acid mediated through selective COX inhibition: Computational and experimental data. Inflammation 2014, 37, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Petersen, K.N.; Thomasen, G.; Christensen, S.B. Isolation of linolenic and α-linolenic acids as COX-1 and -2 inhibitors in Rose Hip. Phytother. Res. 2008, 22, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A., Jr.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kγ/AKT/nNos/NO/Katp signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar]
- Khan, G.R.; Scheinmann, F. Some recent advances in physical methods for analysis and characterization of polyunsaturated fatty acids. Prog. Chem. Fats Lipids 1978, 15, 343–367. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advances in Lipid Methodology; Christie, W.W., Ed.; Oil Press: Dundee, UK, 1993; pp. 69–111. [Google Scholar]
- Miller, L.C.; Tainter, M.L. Estimation of the LD50 and its error by means of logarithmic probit graph paper. Proc. Soc. Exp. Biol. Med. 1994, 57, 261–264. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Acute Oral Toxicity; Environmental Health and Safety Monograph Series on Testing and Assessment No 24; Environment Directorate OECD: Paris, France, 2000. [Google Scholar]
- OECD. Repeated Dose 28-Day Oral Toxicity Study in Rodents; Environment Health and Safety Monograph Series on Testing and Assessment No. 407; Environment Directorate OECD: Paris, France, 1995. [Google Scholar]
- Koster, R.; Anderson, M.; de Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–418. [Google Scholar]
- MacDonald, A.D.; Woolfe, G.; Bergel, F.; Morrison, A.L.; Rinderknecht, H. Analgesic action of pethidine derivatives and related compounds. Br. J. Pharmacol. 1946, 1, 4–14. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, A.S.; De Lima, A.B.; Albuquerque, T.L.F.; Silveira, T.S.; Nascimento, J.L.M.d.; Silva, J.K.R.d.; Ribeiro, A.F.; Maia, J.G.S.; Bastos, G.N.T. Antinociceptive Activity and Toxicity Evaluation of the Fatty Oil from Plukenetia polyadenia Mull. Arg. (Euphorbiaceae). Molecules 2015, 20, 7925-7939. https://doi.org/10.3390/molecules20057925
Mota AS, De Lima AB, Albuquerque TLF, Silveira TS, Nascimento JLMd, Silva JKRd, Ribeiro AF, Maia JGS, Bastos GNT. Antinociceptive Activity and Toxicity Evaluation of the Fatty Oil from Plukenetia polyadenia Mull. Arg. (Euphorbiaceae). Molecules. 2015; 20(5):7925-7939. https://doi.org/10.3390/molecules20057925
Chicago/Turabian StyleMota, Amanda S., Anderson B. De Lima, Thayana Lucy F. Albuquerque, Tiago S. Silveira, José Luiz M. do Nascimento, Joyce Kelly R. da Silva, Alcy F. Ribeiro, José Guilherme S. Maia, and Gilmara N. T. Bastos. 2015. "Antinociceptive Activity and Toxicity Evaluation of the Fatty Oil from Plukenetia polyadenia Mull. Arg. (Euphorbiaceae)" Molecules 20, no. 5: 7925-7939. https://doi.org/10.3390/molecules20057925