Nutritional and Biochemical Profiling of Leucopaxillus candidus (Bres.) Singer Wild Mushroom
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical composition of L. candidus Fruiting Body
| Component | Leucopaxillus candidus |
|---|---|
| Moisture (g/100 g) | 90 ± 0.4 |
| Fat (g/100 g) | 1.8 ± 0.1 |
| Proteins (g/100 g) | 20 ± 0.2 |
| Ash (g/100 g) | 8.2 ± 0.2 |
| Carbohydrates (g/100 g) | 70 ± 1 |
| Energy (kcal/100 g) | 376 ± 1 |
| Fructose (g/100 g) | 0.35 ± 0.01 |
| Mannitol (g/100 g) | 5.6 ± 0.1 |
| Trehalose (g/100 g) | 1.1 ± 0.1 |
| Total sugars (g/100 g) | 7.1 ± 0.1 |
| C16:0 | 8.0 ± 0.7 |
| C18:0 | 2.7 ± 0.1 |
| C18:1n9 | 37 ± 2 |
| C18:2n6 | 49 ± 1 |
| SFA (relative percentage) | 13 ± 1 |
| MUFA (relative percentage) | 37 ± 2 |
| PUFA (relative percentage) | 50 ± 1 |
| α-tocopherol (µg/100 g) | 14 ± 1 |
| β-tocopherol (µg/100 g) | 33 ± 1 |
| γ-tocopherol (µg/100 g) | 3.1 ± 0.1 |
| δ-tocopherol (µg/100 g) | 7.2 ± 0.5 |
| Total tocopherols (µg/100 g) | 58 ± 1 |
| Compound | Leucopaxillus candidus |
|---|---|
| Oxalic acid (g/100 g) | 0.41 ± 0.09 |
| Fumaric acid (g/100 g) | 0.40 ± 0.00 |
| Total organic acids (g/100 g) | 0.81 ± 0.10 |
| p-Hydroxybenzoic acid (µg/100 g) | 0.71 ± 0.05 |
| p-Coumaric acid (µg/100 g) | 0.58 ± 0.05 |
| Total phenolic acids (µg/100 g) | 1.3 ± 0.1 |
| Cinnamic acid (µg/100 g) | 0.12 ± 0.01 |
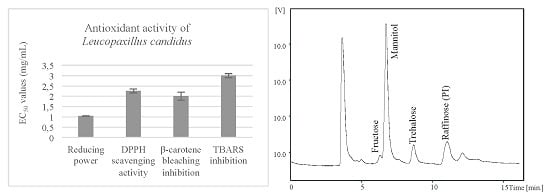
2.2. Antioxidant Activity of the Methanolic Extracts and Confirmation of Non-Toxicity
| Antioxidant Activity | Assay | Leucopaxillus candidus |
|---|---|---|
| Reducing power | Folin-ciocalteu (mg GAE/g extract) | 20 ± 1 |
| Ferricyanide/Prussian blue (EC50; mg/mL) | 1.04 ± 0.01 | |
| Radical scavenging activity | DPPH scavenging activity (EC50; mg/mL) | 2.3 ± 0.1 |
| Lipid peroxidation inhibition | β-carotene/linoleate (EC50; mg/mL) | 2.0 ± 0.4 |
| TBARS (EC50; mg/mL) | 3.0 ± 0.3 |
3. Materials and Methods
3.1. Samples
3.2. Standards and Reagents
3.3. Chemical Composition of L. candidus Fruiting Body
3.4. Bioactivity of L. candidus Methanolic Extract
3.5. Toxicity for Porcine Liver Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Phillips, R. Mushrooms and other Fungi of North America, 2nd ed.; Firefly Books Ltd.: New York, NY, USA, 2005. [Google Scholar]
- Martínez de Aragón, J. Producción de Esporocarpos de Hongos Ectomicorrícicos y Valoración Socioeconómica, Respuesta de Estas Comunidades a Incendios Forestales. Ph.D. Thesis, Escola Tècnica Superior d’Enginyeria Agraria, University de Lleida, Lleida, Spain, 2005. [Google Scholar]
- Benguría, R.L. Mil Setas Ibericas; Diputacíon Foral de Bizkaia: Biskay, Spain, 1985. [Google Scholar]
- Román, M.; Boa, E. Collection, markting and cultivation of edible fungi in Spain. Micol. Aplic. Int. 2004, 16, 25–33. [Google Scholar]
- Tedesco, G.; Galli, R.; Carraro, L. An electrophoretic approach to Basidiomycetes taxonomy: Intraspecific variability, varieties, ecological influences. Plant Biosyst. 2009, 143, 301–310. [Google Scholar] [CrossRef]
- Vizzini, A.; Ercole, E.; Contu, M. A contribution to the ITS-LSU phylogeny of the genus Leucopaxillus (/tricholomatoid clade, Agaricales), with three new genera and notes on Porpoloma. Mycosphere 2012, 3, 79–90. [Google Scholar] [CrossRef]
- Weber, R.; Kuhn, A.; Anke, H. Soil-borne Penicilliun ssp. and other microfungi as efficient degraders of the explosive RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine). Mycol. Prog. 2003, 2, 83–93. [Google Scholar] [CrossRef]
- Caiping, L.; Baohua, P.; Yuhau, Y.; Yigong, Z. A preliminary study on the biological characteristics of Leucopaxillus candidus mycelia. Acta Edulis Fungi 2004, 11, 24–29. [Google Scholar]
- Ahmed, M.; Keeping, J.W.; Macrides, T.A.; Thaller, V. Natural Acetylenes. Part 54. Polyacetylenes from fungal cultures of some Tricholomataceae and Corticiaceae species. J. Chem. Soc. Perkin Trans. 1978, 1, 1487–1489. [Google Scholar] [CrossRef]
- Oliveira, P. Mushroom poisoning. Med. Interna 2009, 16, 253–363. [Google Scholar]
- Kalac, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Mattilla, P.; Suonpää, K.; Piironen, V. Functional properties of edible mushrooms. Nutrition 2000, 16, 694–696. [Google Scholar] [CrossRef]
- Kumari, B.; Atri, N.S. Nutritional and nutraceutical potential of wild edible macrolepiotoid mushrooms of north India. Int. J. Pharm. Pharm. Sci. 2014, 6, 200–204. [Google Scholar]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushroom and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Baptista, P.; Correia, D.M.; Casal, S.; Oliveira, B.; Ferreira, I.C.F.R. Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Hagiwara, S.; Takahashi, M.; Shen, Y.; Kaihou, S.; Tomiyama, T.; Yazawa, Y.; Tamai, Y.; Sin, Y.; Kazusaka, A.; Terazawa, M. A phytochemical in the edible Tamigi-take mushroom (Pleurotus cornucopiae), D-mannitol, inhibits ACE activity and lowers the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2005, 69, 1603–1605. [Google Scholar] [CrossRef] [PubMed]
- Thevelein, J.M. Regulation of trehalose metabolism in fungi. Microbiol. Rev. 1984, 48, 42–59. [Google Scholar] [PubMed]
- Carpenter, J.F.; Crowe, J.F. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 1989, 28, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome1–3. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [PubMed]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Niki, E.; Traber, M.G. A history of vitamin E. Ann. Nutr. Metab. 2012, 61, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pae, M.; Meydani, S.N.; Wu, D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012, 3, 91–129. [Google Scholar] [PubMed]
- Ribeiro, B.; Andrade, P.B.; Baptista, P.; Barros, L.; Ferreira, I.C.F.R.; Seabra, R.M.; Valentão, P. Leucopaxillus giganteus mycelium: Effect of nitrogen source on organic acids and alkaloids. J. Agric. Food Chem. 2008, 56, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Method. 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen diferent Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.G.; Geissler, C.A. The New Oxford Book of Food Plants; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Silva, B.M.; Andrade, P.B.; Gonçalves, A.C.; Seabra, R.M.; Oliveira, M.B.; Ferreira, M.A. Influence of jam processing upon the contents of phenolics, organic acids and free amino acids in quince fruit (Cydonia oblonga Miller). Eur. Food Res. Technol. 2004, 218, 385–389. [Google Scholar] [CrossRef]
- Son, S.M.; Moon, K.D.; Lee, C.Y. Kinetic study of oxalic acid inhibition on enzymatic browning. J. Agric. Food Chem. 2000, 48, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl,3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendixen, E.; Danielsen, M.; Larsen, K.; Bendixen, C. Advances in porcine genomics and proteomics—A toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct. Genom. 2010, 9, 208–219. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Sample Availability: Samples of this mushrooms are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, V.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Nutritional and Biochemical Profiling of Leucopaxillus candidus (Bres.) Singer Wild Mushroom. Molecules 2016, 21, 99. https://doi.org/10.3390/molecules21010099
Vieira V, Barros L, Martins A, Ferreira ICFR. Nutritional and Biochemical Profiling of Leucopaxillus candidus (Bres.) Singer Wild Mushroom. Molecules. 2016; 21(1):99. https://doi.org/10.3390/molecules21010099
Chicago/Turabian StyleVieira, Vanessa, Lillian Barros, Anabela Martins, and Isabel C. F. R. Ferreira. 2016. "Nutritional and Biochemical Profiling of Leucopaxillus candidus (Bres.) Singer Wild Mushroom" Molecules 21, no. 1: 99. https://doi.org/10.3390/molecules21010099