A Potential Mechanism for the Anti-Apoptotic Property of Koumine Involving Mitochondrial Pathway in LPS-Mediated RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Results
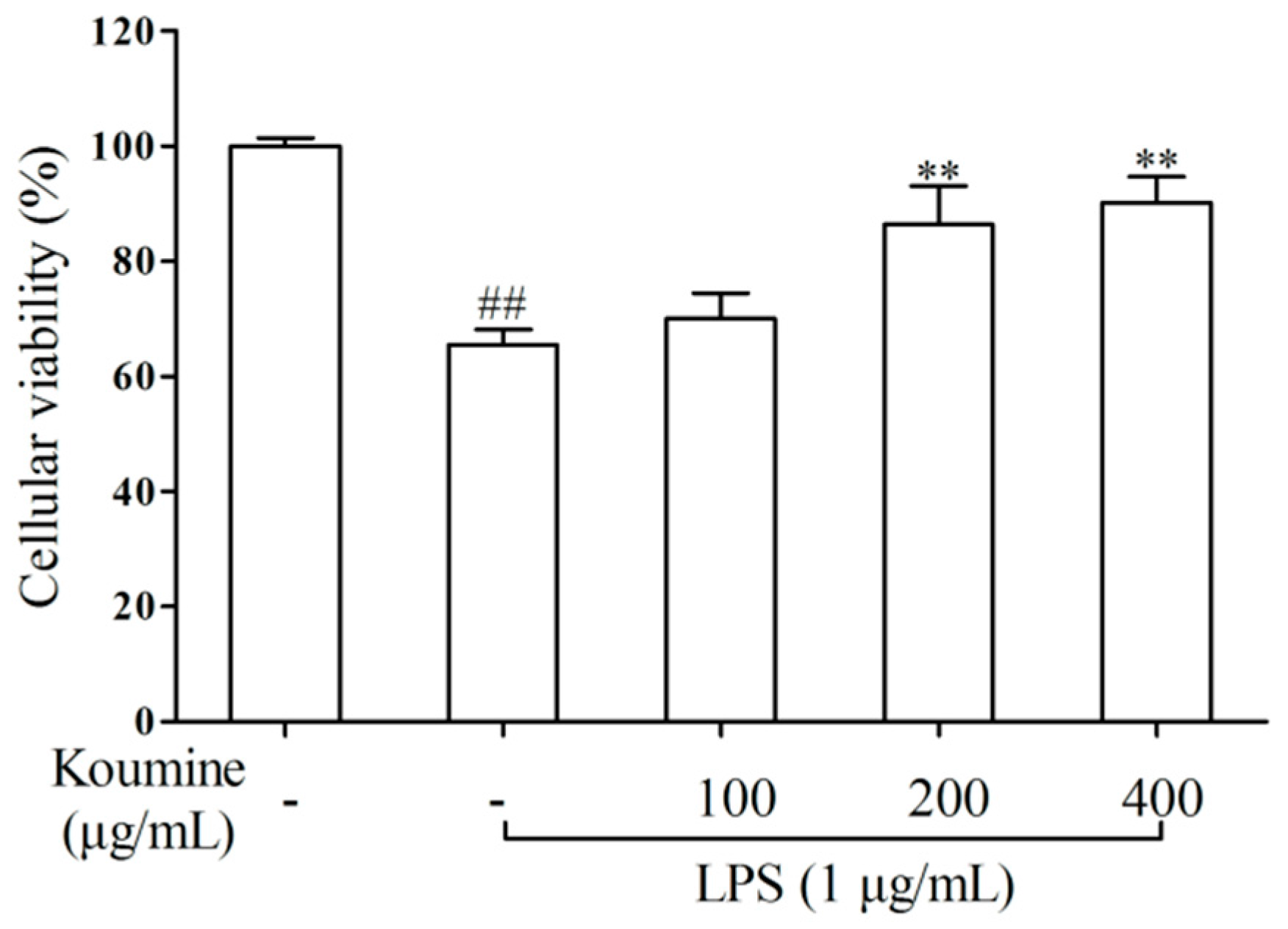
2.1. Cell Viability
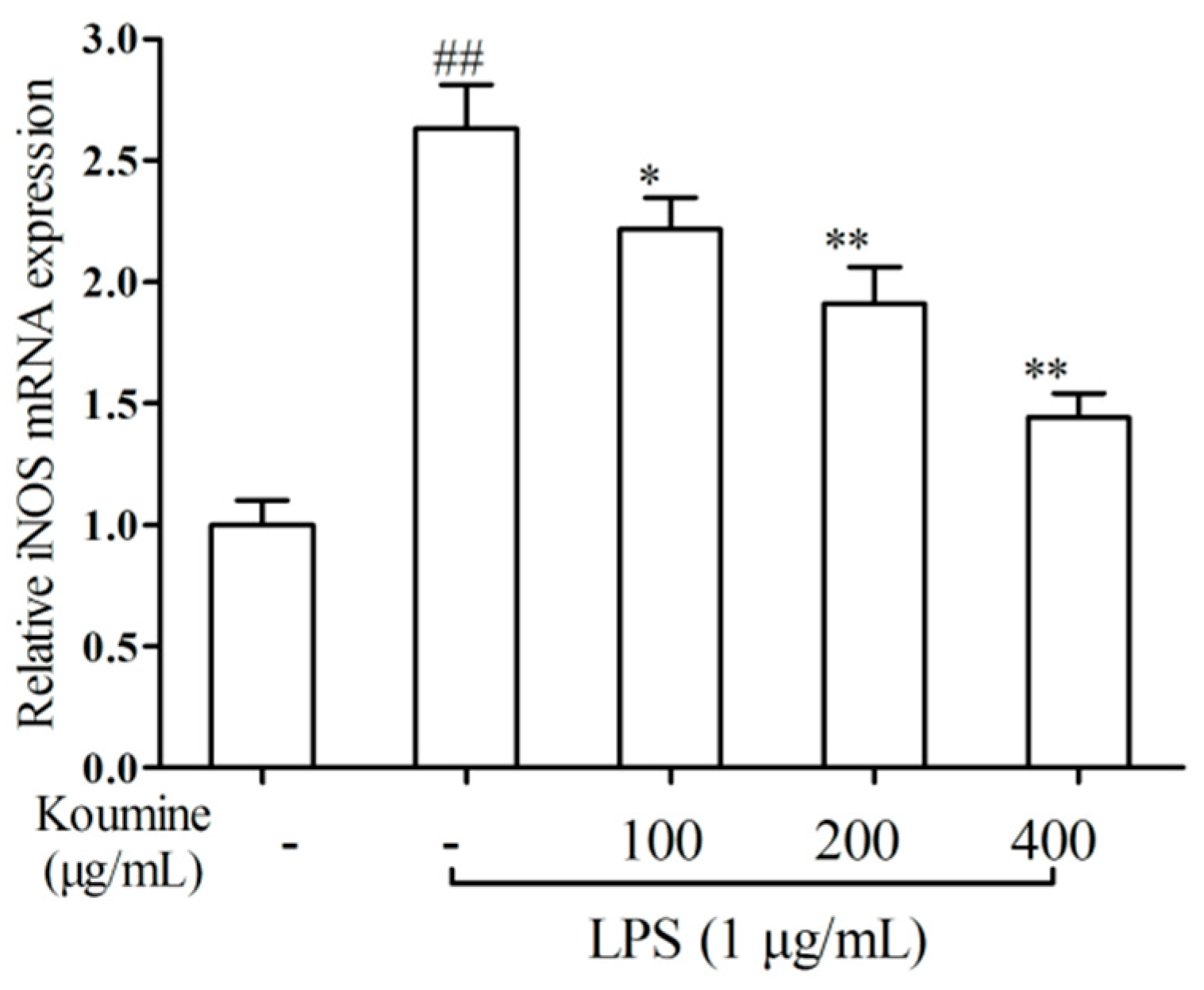
2.2. iNOS qRT-PCR Assay
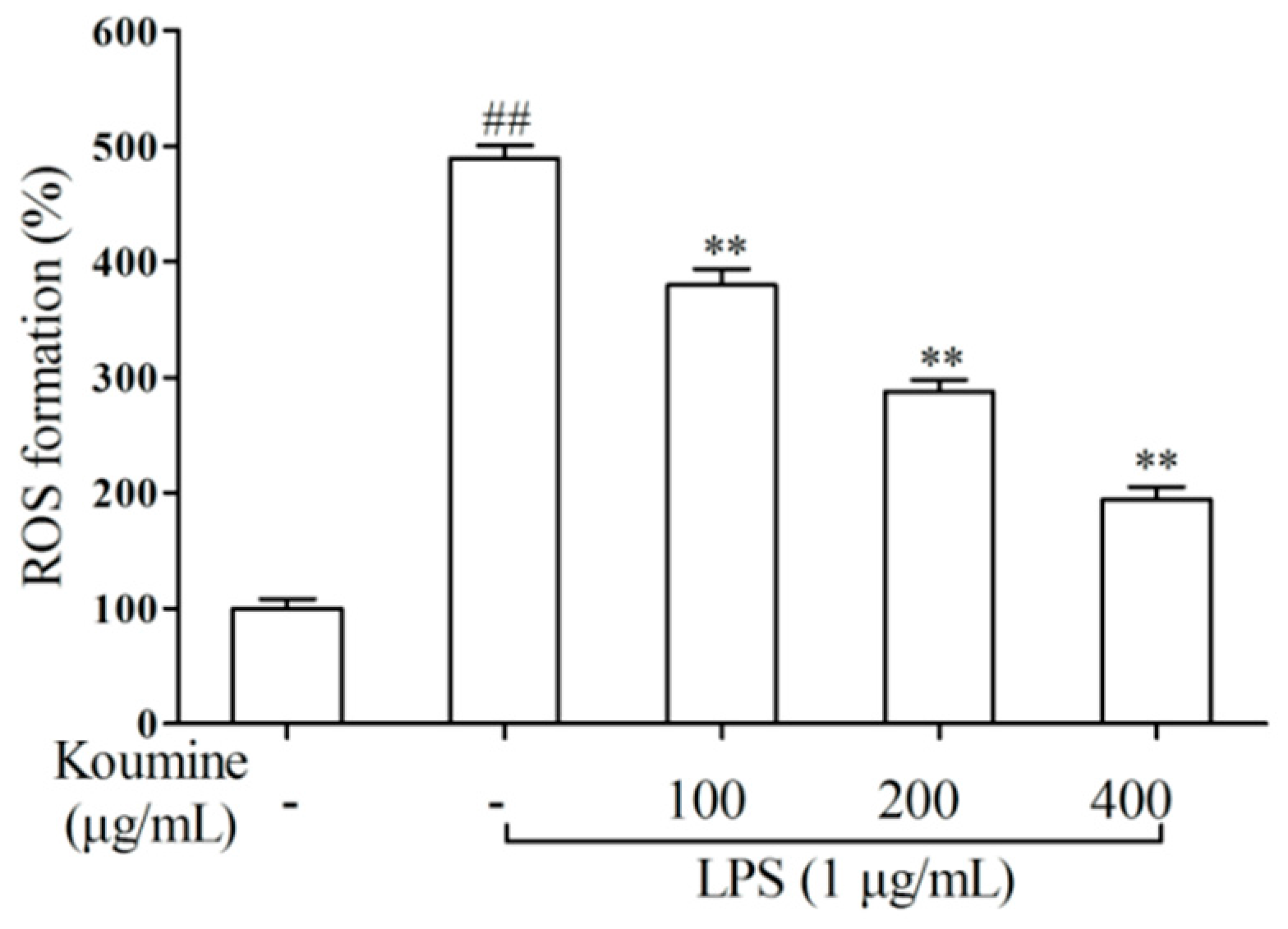
2.3. ROS Assay
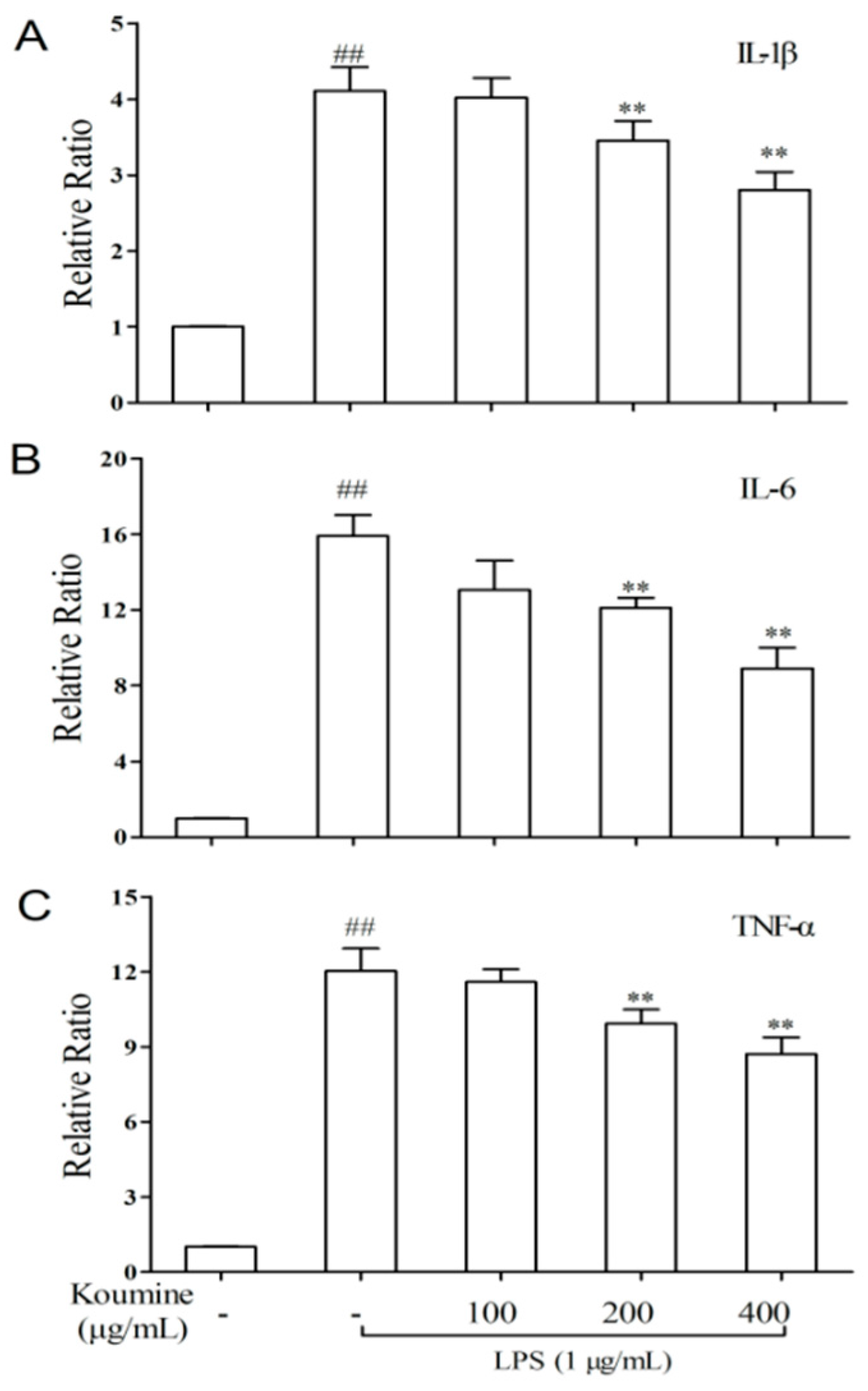
2.4. TNF-α, IL-1β, and IL-6 mRNA Expression
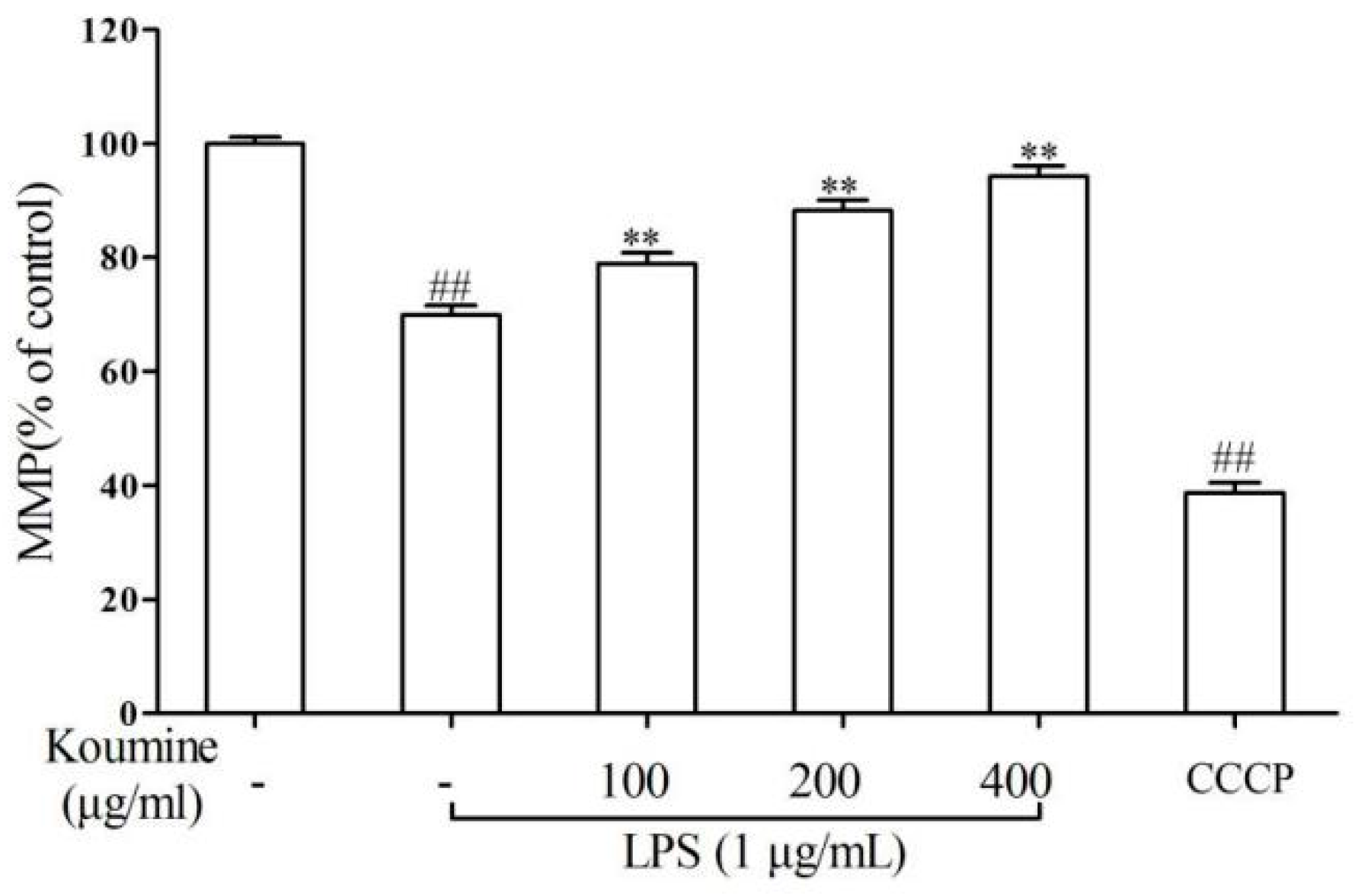
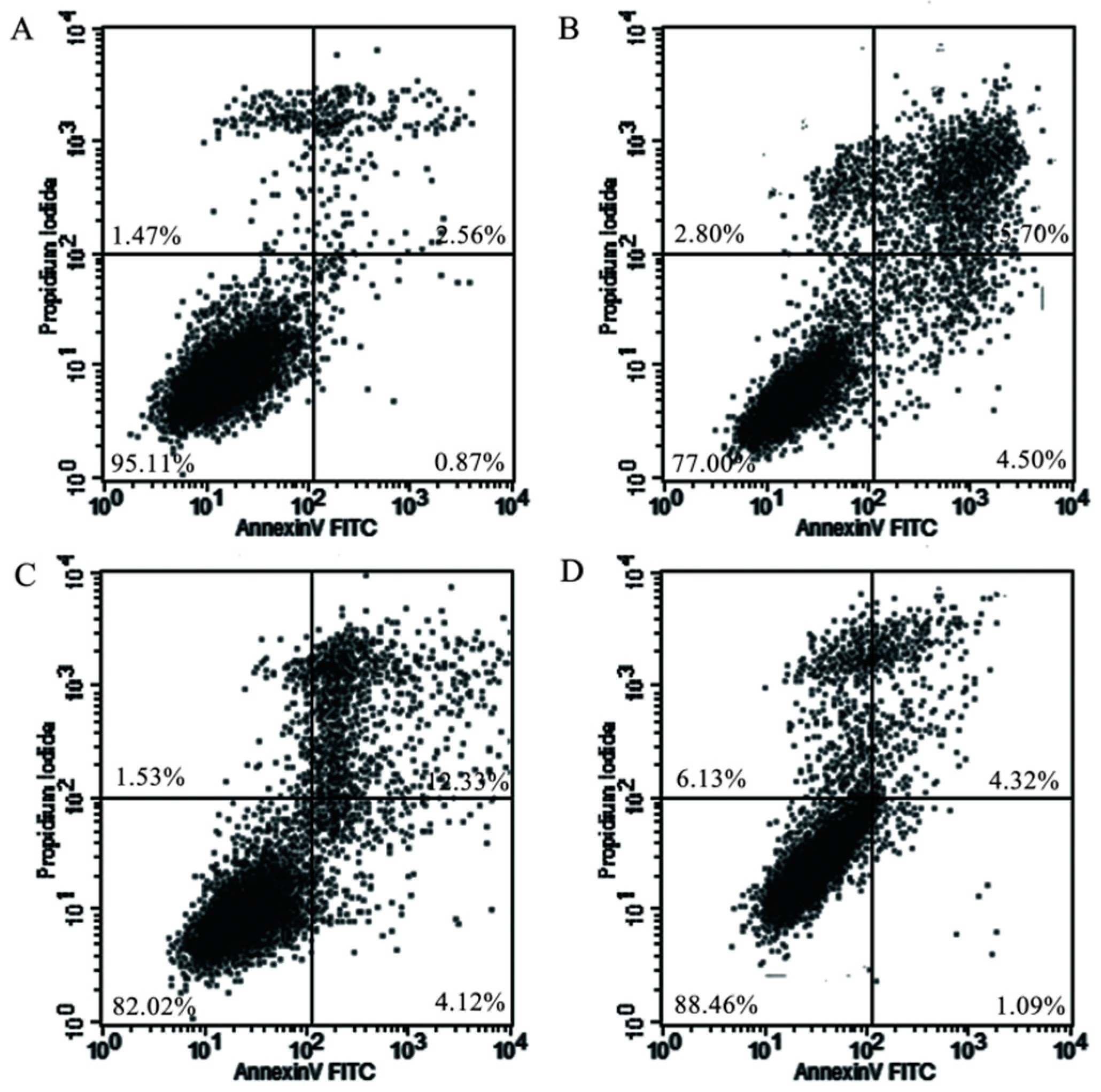
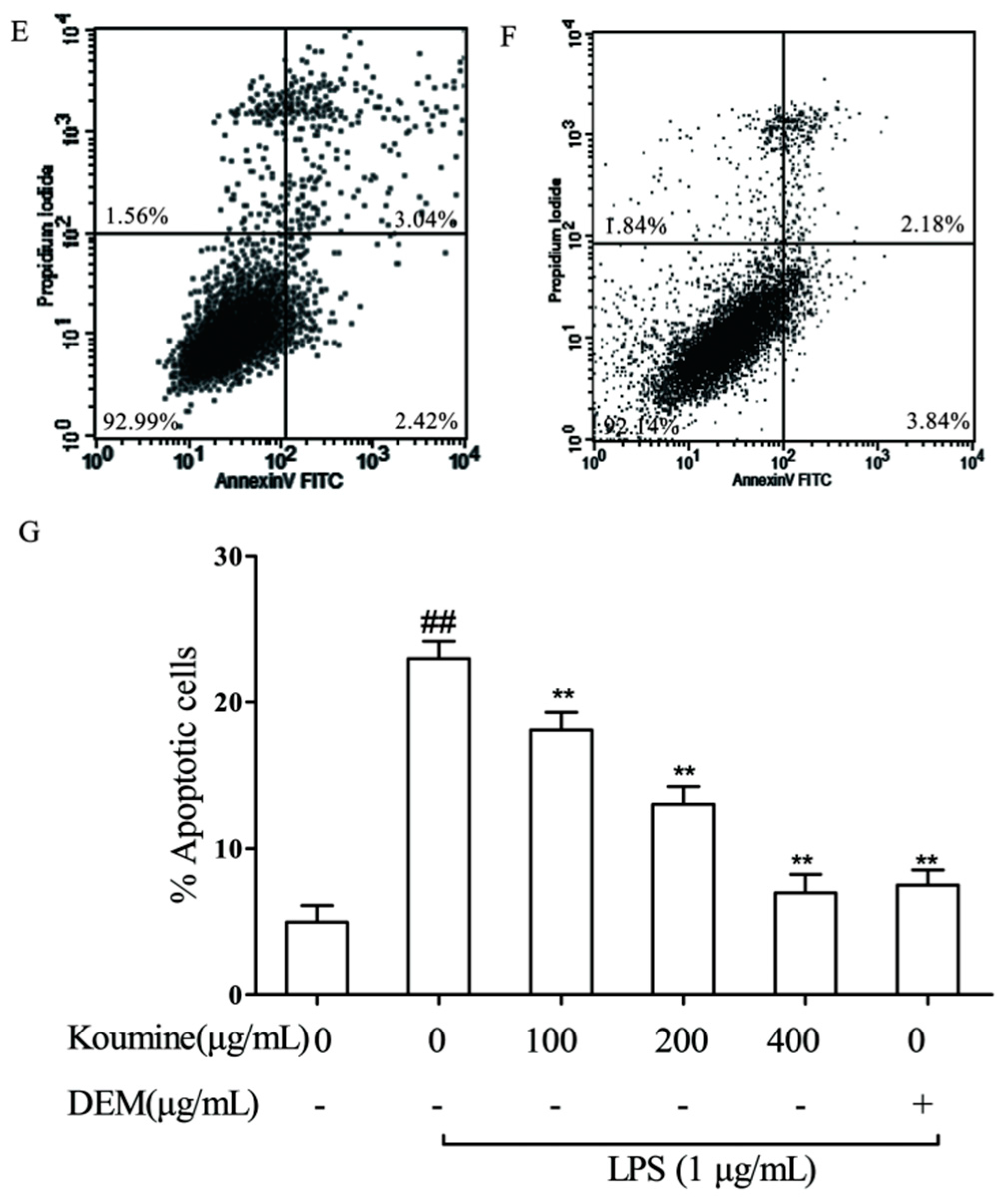
2.5. Mitochondrial Injury and Cell Apoptosis
2.6. Analysis of Caspase Activities
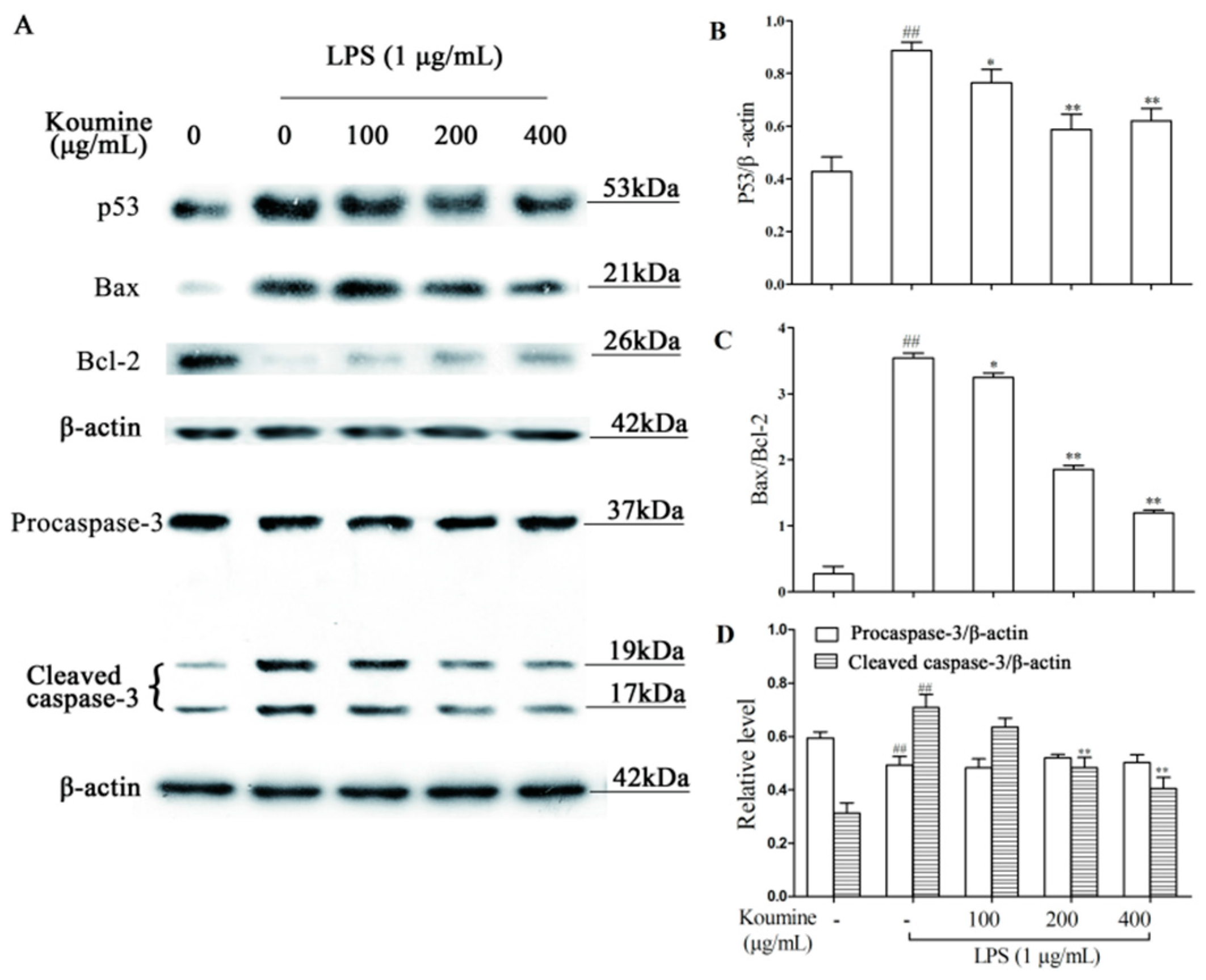
2.7. Regulation of Apoptosis-Related Proteins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTT Assay
4.4. Determination of ROS
4.5. iNOS, IL-1β, IL-6, and TNF-α qRT-PCR Assay
4.6. Mitochondrial Membrane Potential
4.7. Flow Cytometry
4.8. Caspase-3 and -9 Activities
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar] [PubMed]
- Wyns, H.; Plessers, E.; de Backer, P.; Meyer, E.; Croubels, S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet. Immunol. Immunopathol. 2015, 166, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Sama, D.; Calatroni, A. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J. Cell. Biochem. 2009, 106, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.B.; Wong, F.; Harlan, J.M.; Chaudhary, P.M.; Hood, L.; Karsan, A. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J. Biol. Chem. 1998, 237, 20185–20188. [Google Scholar] [CrossRef]
- Fielhaber, J.A.; Carroll, S.F.; Dydensborg, A.B.; Shourian, M.; Triantafillopoulos, A.; Harel, S.; Hussain, S.N.; Bouchard, M.; Qureshi, S.T.; Kristof, A.S. Inhibition of mammalian target of rapamycin augments lipopolysaccharide-induced lung injury and apoptosis. J. Immunol. 2012, 188, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Namisaki, T.; Yoshiji, H.; Kojima, H.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Sakurai, S.; Yanase, K.; Kitade, M.; Yamazaki, M.; et al. Salvage effect of the vascular endothelial growth factor on chemically induced acute severe liver injury in rats. J. Hepatol. 2006, 44, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, Q.H.; Wu, Q.; Li, F.; Lu, Y.F.; Shi, J.S. Alkaloids enriched extract from Dendrobium nobile Lindl. attenuates tau protein hyperphosphorylation and apoptosis induced by lipopolysaccharide in rat brain. Phytomedicine 2014, 21, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.S.; Liu, Y.Q.; Li, M.; Li, B.S.; Xu, Y. Protective effects and its mechanisms of total alkaloids from rhizome Coptis chinensis on Helicobacter pylori LPS induced gastric lesion in rats. China J. Chin. Mater. Med. 2007, 32, 1333–1336. [Google Scholar]
- Dutt, V.; Thakur, S.; Dhar, V.J.; Sharma, A. The genus Gelsemium: An update. Pharmacogn. Rev. 2010, 4, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Shi, D.M.; Yu, C.X. Pharmacognostical study on the Gelsemium elegans Benth. from Fuzhou. Strait Pharm. J. 2008, 20, 62–64. [Google Scholar]
- Wang, Y.H.; Wu, S.S.; Chen, Z.C.; Zhang, H.; Zhao, W.L. Inhibitory effects of cytochrome P450 enzymes CYP1A2, CYP2A6, CYP2E1 and CYP3A4 by extracts and alkaloids of Gelsemium elegans roots. J. Ethnopharmacol. 2015, 166, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.R.; Qin, R.; Cai, J.; Chi, D.B. Antitumor activity of koumine in vitro and vivo. Pharmacol. Clin. Chin. Mater. Med. 2006, 22, 6–8. [Google Scholar]
- Liu, M.; Huang, H.H.; Yang, J.; Su, Y.P.; Lin, H.W.; Lin, L.Q.; Liao, W.J.; Yu, C.X. The active alkaloids of Gelsemium elegans benth. are potent anxiolytics. Psychopharmacology 2013, 225, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Q.; Qiu, C.Z.; Zheng, L.Z. Analgesic effect and no physical dependence of Gelsemium elegans Benth. Pharmacol. Clin. Chin. Mater. Med. 1988, 4, 24–28. [Google Scholar]
- Xu, Y.; Qiu, H.Q.; Liu, H.; Liu, M.; Huang, Z.Y.; Yang, J.; Su, Y.P.; Yu, C.X. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol. Biochem. Behav. 2012, 101, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, C.; Spallotta, F.; Martelli, F.; Valente, S.; Mai, A.; Zeiher, A.M.; Gaetano, C. Oxidative stress and epigenetic regulation in ageing and age-related diseases. Int. J. Mol. Sci. 2013, 14, 17643–17663. [Google Scholar] [CrossRef] [PubMed]
- Noworyta-Sokołowska, K.; Górska, A.; Gołembiowska, K. LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharm. Rep. 2013, 65, 863–869. [Google Scholar] [CrossRef]
- Park, C.M.; Park, Y.J.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Cuong, T.D.; Hung, T.M.; Lee, I.; Na, M.K.; Kim, J.C.; Ryoo, S.; Lee, J.H.; Choi, J.S.; Woo, M.H.; et al. Alkaloids from Chelidonium majus and their inhibitory effects on LPS-induced NO production in RAW264. 7 cells. Bioorg. Med. Chem. Lett. 2011, 21, 6960–6963. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kang, Y.J.; Park, M.K.; Lee, Y.S.; Seo, H.G.; Kim, T.S.; Kim, C.H.; Chang, K.C. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci. 2003, 73, 1401–1412. [Google Scholar] [CrossRef]
- Wang, Z.R.; Huang, C.Q.; Zhang, Z.Y.; Zhang, L.L.; Lin, J.M. Effect of koumine on proliferation of murine CD4+ T cells purified by magnetic-activated cell sorting in vitro. J. First Mil. Med. Univ. 2005, 25, 562–564. [Google Scholar]
- Mchugh, S.M.; Rifkin, I.R.; Deighton, J.; Wilsion, A.B.; Lachamnn, P.J.; Lockwood, C.M. The immunosuppressive drug thalidomide induces T helper cell type 2 (Th2) and concomitantly inhibits Thl cytokine production in mitogen- and antigen-stimulated human peripheral blood mononuclear cell cultures. Clin. Exp. Immunol. 1995, 99, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Cooper, M.A.; Nuovo, G.J.; Cella, M.; Facchetti, F.; Colonna, M.; Caligiuri, M.A. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell–derived IL-2: A potential new link between adaptive and innate immunity. Blood 2003, 101, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Sittrich, A.B.; Haftmann, C.; Sgouroudis, E.; Kühl, A.A.; Hegazy, A.N.; Panse, I.; Riedel, R.; Flossdorf, M.; Dong, J.; Fuhrmann, F.; et al. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nat. Immunol. 2010, 11, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Vermi, W.; Parodi, M.; Pietra, G.; Manzini, C.; Queirolo, P.; Lonardi, S.; Augugliaro, R.; Moretta, A.; Facchetti, F.; et al. Melanoma cells become resistant to NK-cell-mediated killing when exposed to NK-cell numbers compatible with NK-cell infiltration in the tumor. Eur. J. Immunol. 2012, 42, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Melencio, L.; Mckallip, R.J.; Guan, H.; Ramakrishnan, R.; Jain, R.; Nagarkatti, P.S.; Nagarkatti, M. Role of CD4+CD25+ T regulatory cells in IL-2-induced vascular leak. Int. Immunol. 2006, 18, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.C.; Bhatt, R.S.; Parikh, S.M.; Patel, P.; Seery, V.; Mcdermott, D.F.; Atkins, M.B.; Sukhatme, V.P. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clin. Cancer Res. 2007, 13, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Singh, N.P.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS ONE 2012, 7, e35650. [Google Scholar] [CrossRef] [PubMed]
- Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. The role of IL-2 in the activation and expansion of regulatory T-cells and the development of experimental autoimmune encephalomyelitis. Immunobiology 2013, 216, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Toren, D.; Barzilay, T.; Tacutu, R.; Lehmann, G.; Muradian, K.K.; Fraifeld, V.E. MitoAge: A database for comparative analysis of mitochondrial DNA, with a special focus on animal longevity. Nucl. Acids Res. 2016, 44, D1262–D1265. [Google Scholar] [CrossRef] [PubMed]
- Novoderezhkina, E.A.; Zhivotovsky, B.D.; Gogvadze, V.G. Induction of unspecific permeabilization of mitochondrial membrane and its role in cell death. Mol. Biol. 2016, 50, 51–68. [Google Scholar] [CrossRef]
- Barnwal, B.; Karlberg, H.; Mirazimi, A.; Tan, Y.J. The non-structural protein of crimean-congo hemorrhagic fever virus disrupts the mitochondrial membrane potential and induces apoptosis. J. Biol. Chem. 2016, 291, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Deckwerth, T.L.; Easton, R.M.; Knudson, C.M.; Korsmeyer, S.J.; Johnson, E.M. Placement of the BCL2 family member BAX in the death pathway of sympathetic neurons activated by trophic factor deprivation. Exp. Neurol. 1998, 152, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Mignotte, B.; Vayssière, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Cohen, H.B.; Mosser, D.M. Extrinsic and intrinsic control of macrophage inflammatory responses. J. Leukoc. Biol. 2013, 94, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.; Tabas, I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 2009, 50, S382–S387. [Google Scholar] [CrossRef] [PubMed]
- Langford, M.P.; McGee, D.J.; Ta, K.H.; Redens, T.B.; Texada, D.E. Multiple caspases mediate acute renal cell apoptosis induced by bacterial cell wall components. Ren. Fail. 2011, 33, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Jin, S.; Pabon, K.; Scotto, K.W. A role for ABCG2 beyond drug transport: Regulation of autophagy. Autophagy 2016, 12, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Klionsky, D.J. Rph1 mediates the nutrient-limitation signaling pathway leading to transcriptional activation of autophagy. Autophage 2016, 11, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Hinića, V.; Brodarda, I.; Thomanna, A.; Cvetnićc, Ž.; Makayad, P.V.; Freyb, J.; Abril, C. Novel identification and differentiation of Brucella melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae suitable for both conventional and real-time PCR systems. J. Microbiol. Meth. 2008, 75, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.-H.; Liang, Z.-E.; Wu, J.; Yi, J.-E.; Chen, X.-J.; Sun, Z.-L. A Potential Mechanism for the Anti-Apoptotic Property of Koumine Involving Mitochondrial Pathway in LPS-Mediated RAW 264.7 Macrophages. Molecules 2016, 21, 1317. https://doi.org/10.3390/molecules21101317
Yuan Z-H, Liang Z-E, Wu J, Yi J-E, Chen X-J, Sun Z-L. A Potential Mechanism for the Anti-Apoptotic Property of Koumine Involving Mitochondrial Pathway in LPS-Mediated RAW 264.7 Macrophages. Molecules. 2016; 21(10):1317. https://doi.org/10.3390/molecules21101317
Chicago/Turabian StyleYuan, Zhi-Hang, Zeng-Enni Liang, Jing Wu, Jin-E Yi, Xiao-Jun Chen, and Zhi-Liang Sun. 2016. "A Potential Mechanism for the Anti-Apoptotic Property of Koumine Involving Mitochondrial Pathway in LPS-Mediated RAW 264.7 Macrophages" Molecules 21, no. 10: 1317. https://doi.org/10.3390/molecules21101317




