Natural and Synthetic Coumarins with Effects on Inflammation
Abstract
:1. Introduction
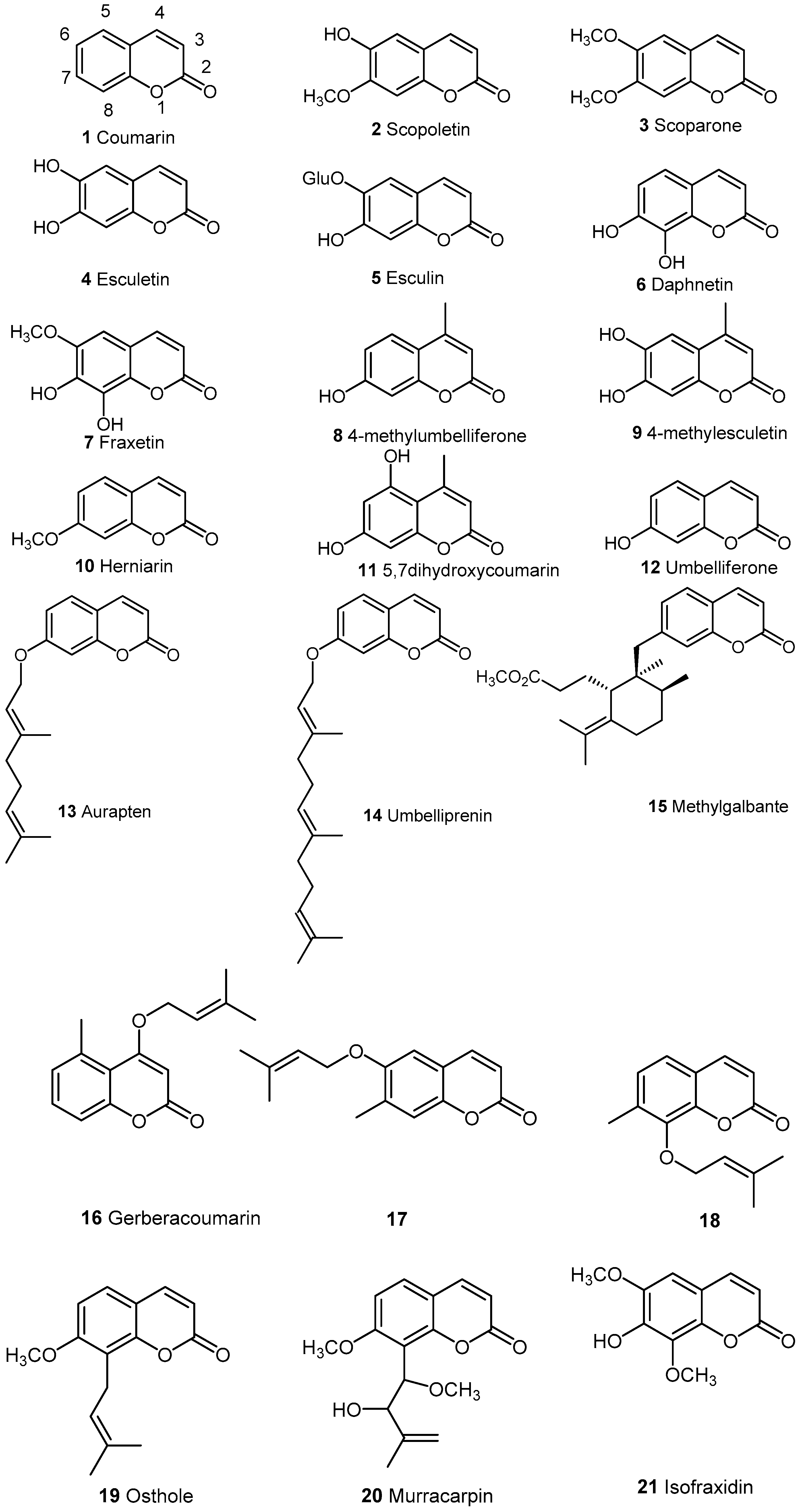
2. Naturally Occurring Coumarins
2.1. Simple Coumarins
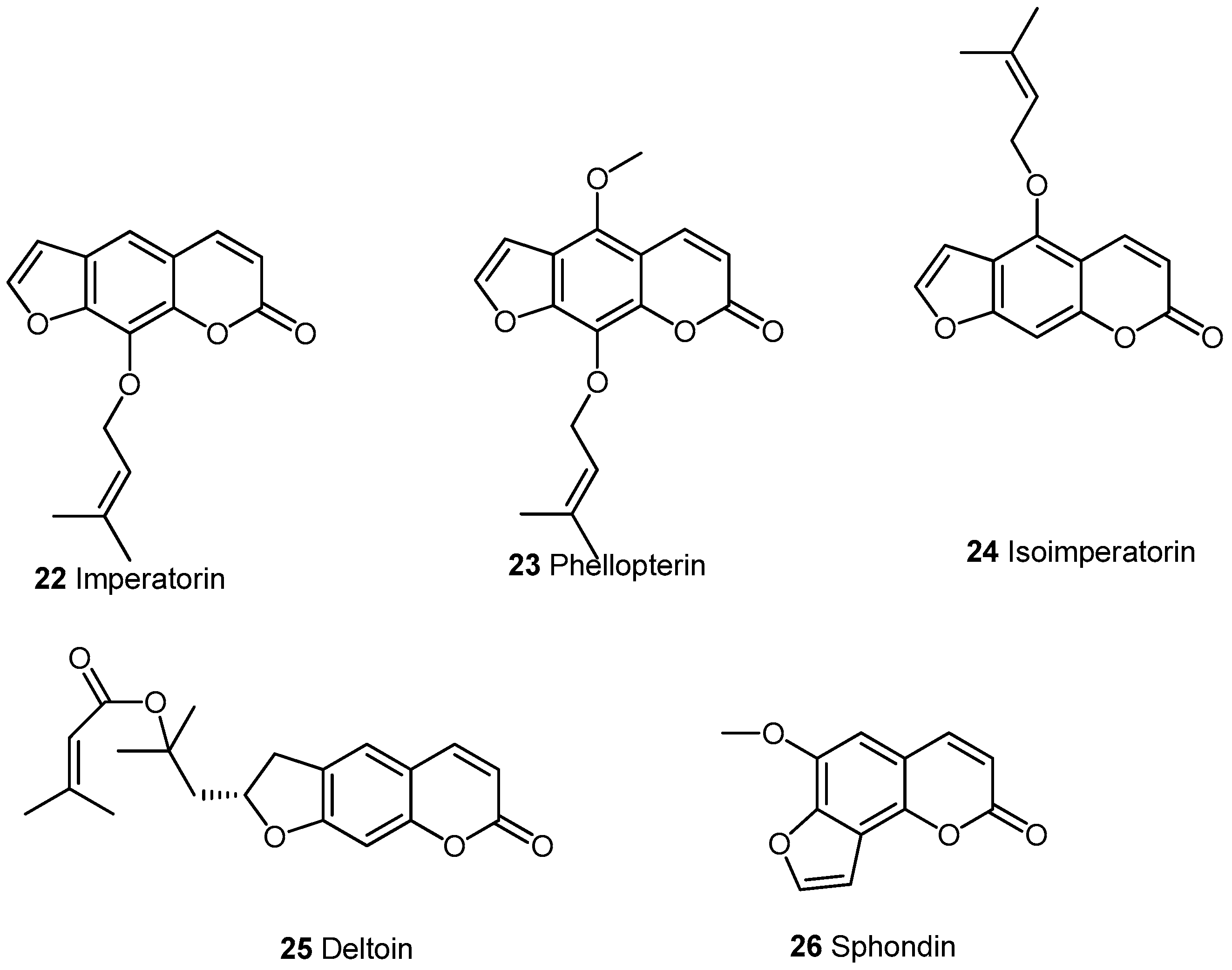
2.2. Condensed Coumarins
2.2.1. Furocoumarins
2.2.2. Pyranocoumarins
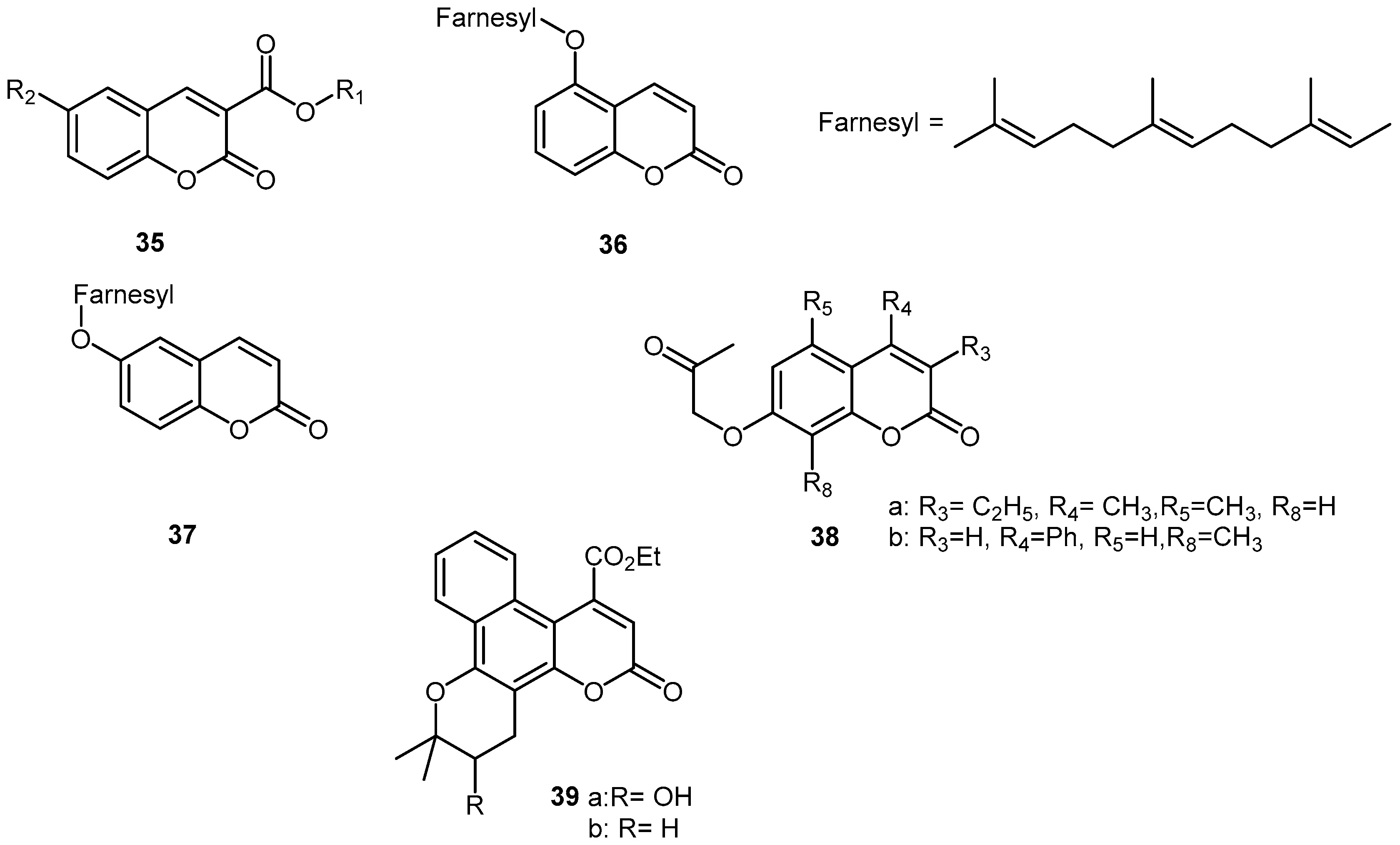
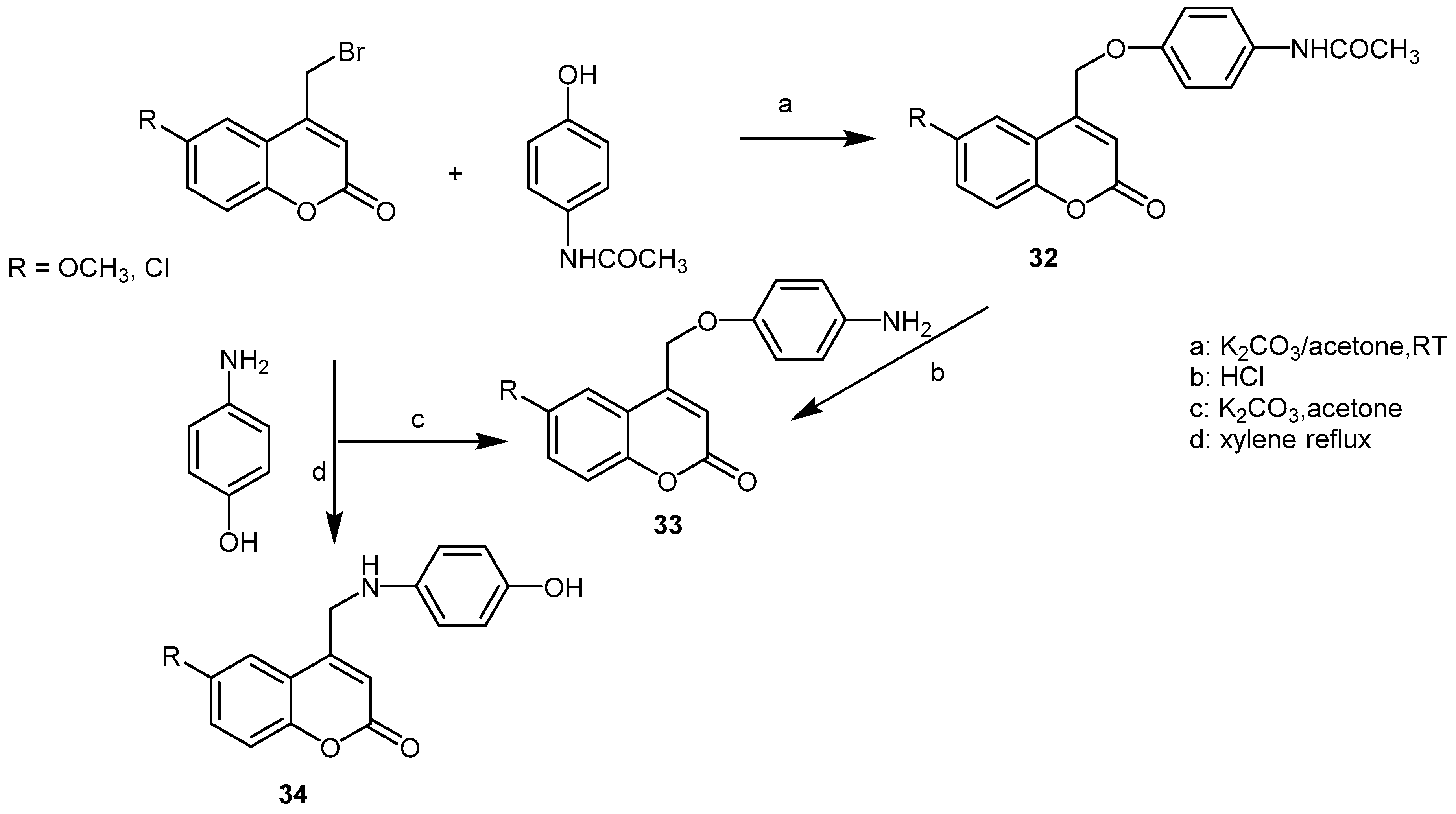
3. Synthetic Coumarins and Pyranocoumarins
4. Conclusions
Conflicts of Interest
References
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Piller, N.B. A comparison of the effectiveness of some anti-inflammatory drugs on thermal oedema. Br. J. Exp. Pathol. 1975, 56, 554–559. [Google Scholar] [PubMed]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Kim, H.P. Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents. Arch. Pharm. Res. 2011, 34, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, Y.; Li, W.; Zhang, H.; Wang, X.; Mu, Q.; He, Z.; Yao, H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015, 29, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.; Forder, R.A.; de las Heras, B.; Lobo, I.B.; Payá, M. Inhibitory activity of a series of coumarins on leukocyte eicosanoid generation. Agents Actions 1994, 42, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Fu, Y.; Li, S.; Tu, L.; Zeng, X.; Kuang, N. Regulatory effect of daphnetin, a coumarin extracted from Daphne odora, on the balance of Treg and Th17 in collagen-induced arthritis. Eur. J. Pharmacol. 2011, 670, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, H.; Ying, H.; Yu, Y.; Chen, D.; Ge, W.; Shi, L. Daphnetin attenuates microglial activation and proinflammatory factor production via multiple signaling pathways. Int. Immunopharmacol. 2014, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gholami, O.; Shamsara, J. Comparison of the cytotoxic effects of umbelliprenin and auraptene. Int. J. Pharm. Pharm. Sci. 2015, 8, 1–4. [Google Scholar]
- Shakeri, A.; Iranshahy, M.; Iranshahi, M. Biological properties and molecular targets of umbelliprenin—A mini-review. J. Asian Nat. Prod. Res. 2014, 16, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Murata, T.; Sugiura, A.; Ito, C.; Iranshahi, M.; Hikita, K.; Kaneda, N. Methyl galbanate, a novel inhibitor of nitric oxide production in mouse macrophage RAW264.7 cells. J. Nat. Med. 2011, 65, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Azelmat, J.; Fiorito, S.; Taddeo, V.A.; Genovese, S.; Epifano, F.; Grenier, D. Synthesis and evaluation of antibacterial and anti-inflammatory properties of naturally occurring coumarins. Phytochem. Lett. 2015, 13, 399–405. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A Review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.C.Y.; Yao, Q.S.; Li, B.H.P. Inhibition of lens protein-, endotoxin- and interleukin-1-induced ocular inflammation with osthole and some other non-steroidal anti-inflammatory agents. Inflammopharmacology 1993, 2, 5–13. [Google Scholar] [CrossRef]
- Wu, L.-H.; Liu, Z.-W.; Zeng, J.; Xu, R.-A. Anti-inflammation and analgesic activities of coumarins compounds from the leaves of Murraya Exotica (L.). Chin. J. Spectrosc. Lab. 2011, 6, 2999–3003. [Google Scholar]
- Nahar, P.P.; Driscoll, M.V.; Li, L.; Slitt, A.L.; Seeram, N.P. Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. J. Funct. Foods 2014, 6, 126–136. [Google Scholar] [CrossRef]
- Curini, M.; Cravotto, G.; Epifano, F.; Giannone, G. Chemistry and biological activity of natural and synthetic prenyloxycoumarins. Curr. Med. Chem. 2006, 13, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kim, C.Y.; Song, D.-G.; Pan, C.-H.; Cha, K.H.; Lee, D.-U.; Um, B.-H. Rapid identification of furanocoumarins in Angelica dahurica using the online LC-MMR-MS and their nitric oxide inhibitory activity in RAW 264.7 cells. Phytochem. Anal. PCA 2010, 21, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-J.; Deng, J.-S.; Liao, J.-C.; Hou, W.-C.; Wang, S.-Y.; Sung, P.-J.; Kuo, Y.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis. J. Agric. Food Chem. 2012, 60, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.-G.; Wei, W.; Yang, X.-W.; Zhang, Y.-B.; Xu, W.; Gong, N.-B.; Lü, Y.; Wang, F.-F. New coumarins from the roots of Angelica dahurica var. formosana cv. Chuanbaizhi and their inhibition on NO production in LPS-activated RAW264.7 cells. Fitoterapia 2012, 101, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, J.; Jiang, L.; Duan, L.; Huo, M.; Chen, N.; Zhong, W.; Wassy, L.; Yang, Z.; Feng, H. Imperatorin attenuates LPS-induced inflammation by suppressing NF-κB and MAPKs activation in RAW 264.7 macrophages. Inflammation 2012, 35, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.J.; Lee, D.U.; Shin, H.M. Anti-inflammatory and antioxidant effects of coumarins isolated from Foeniculum vulgare in lipopolysaccharide-stimulated macrophages and 12-O-tetradecanoylphorbol-13-acetate-stimulated mice. Immunopharmacol. Immunotoxicol. 2015, 37, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Shin, K.H.; Lim, S.S.; Kwak, J.H.; Zee, O.; Ishihara, K.; Hirasawa, N.; Seyama, T.; Ohuchi, K. Lead compounds for anti-inflammatory drugs isolated from the plants of the traditional oriental medicine in Korea. Inflamm. Allergy Drug Targets 2008, 7, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-T.; Lee, E.; Park, N.-Y.; Kim, S.-G.; Park, H.-H.; Lee, J.; Lee, Y.J.; Lee, E. Imperatorin Suppresses Degranulation and Eicosanoid Generation in Activated Bone Marrow-Derived Mast Cells. Biomol. Ther. 2015, 23, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-A.; Kim, H.-M.; Jeong, H.-J. Distinct effects of imperatorin on allergic rhinitis: Imperatorin inhibits caspase-1 activity in vivo and in vitro. J. Pharmacol. Exp. Ther. 2011, 339, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chi, G.; Soromou, L.W.; Chen, N.; Guan, M.; Wu, Q.; Wang, D.; Li, H. Preventive effect of Imperatorin on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2012, 14, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-L.; Im, M.; Lee, M.-H.; Roh, S.-S.; Choi, B.W.; Kim, S.J.; Sohn, K.-C.; Lee, Y.; Seo, Y.-J.; Lee, J.-H.; et al. Inhibitory effect of imperatorin on insulin-like growth factor-1-induced sebum production in human sebocytes cultured in vitro. Life Sci. 2016, 144, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Hsiao, G.; Wang, C.-C.; Lee, Y.-L. Imperatorin exerts antiallergic effects in Th2-mediated allergic asthma via induction of IL-10-producing regulatory T cells by modulating the function of dendritic cells. Pharmacol. Res. 2016, 110, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Q.; Song, Y.-L.; Zhu, Z.-X.; Su, C.; Zhang, X.; Wang, J.; Shi, S.-P.; Tu, P.-F. Anti-inflammatory dimeric furanocoumarins from the roots of Angelica dahurica. Fitoterapia 2015, 105, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Jin, M.; Son, J.K.; Chang, H.W. The effects of isoimperatorin isolated from Angelicae dahuricae on cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2008, 31, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Moon, L.; Ha, Y.M.; Jang, H.J.; Kim, H.S.; Jun, M.S.; Kim, Y.M.; Lee, Y.S.; Lee, D.H.; Son, K.H.; Kim, H.J.; et al. Isoimperatorin, cimiside E and 23-O-acetylshengmanol-3-xyloside from Cimicifugae rhizome inhibit TNF-α-induced VCAM-1 expression in human endothelial cells: involvement of PPAR-γ upregulation and PI3K, ERK1/2, and PKC signal pathways. J. Ethnopharmacol. 2011, 133, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chen, L.G.; Yang, L.L. Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Lett. 1999, 145, 151–157. [Google Scholar] [CrossRef]
- Wang, C.C.; Lai, J.E.; Chen, L.G.; Yen, K.Y.; Yang, L.L. Inducible nitric oxide synthase inhibitors of Chinese herbs. Part 2: naturally occurring furanocoumarins. Bioorg. Med. Chem. 2000, 8, 2701–2707. [Google Scholar] [CrossRef]
- Yang, L.L.; Liang, Y.C.; Chang, C.W.; Lee, W.S.; Kuo, C.T.; Wang, C.C.; Lee, H.M.; Lin, C.H. Effects of sphondin, isolated from Heracleum laciniatum, on IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Life Sci. 2002, 72, 199–213. [Google Scholar] [CrossRef]
- Tosun, F.; Kızılay, Ç.A.; Erol, K.; Kılıç, F.S.; Kürkçüoğlu, M.; Can Başer, K.H. Anticonvulsant activity of furanocoumarins and the essential oil obtained from the fruits of Heracleum crenatifolium. Food Chem. 2008, 107, 990–993. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Mehrabani, G.D.; Ahmadi, M.; Ranjbar, R.; Ardebily, M.S.-A. Chemistry, pharmacology and medicinal properties of Heracleum persicum Desf. Ex Fischer: A review. J. Med. Plants Res. 2012, 6, 1813–1820. [Google Scholar]
- Lima, V.; Silva, C.B.; Mafezoli, J.; Bezerra, M.M.; Moraes, M.O.; Mourão, G.S.M.M.; Silva, J.N.; Oliveira, M.C.F. Antinociceptive activity of the pyranocoumarin seselin in mice. Fitoterapia 2006, 77, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shehzad, O.; Cheng, M.-S.; Li, R.-J.; Kim, Y.S. Pharmacological mechanism underlying anti-inflammatory properties of two structurally divergent coumarins through the inhibition of pro-inflammatory enzymes and cytokines. J. Inflamm. Lond. Engl. 2015, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.-J.; Ci, W.; Wang, G.-F.; Zhang, J.-Y.; Wu, S.-Y.; Xu, W.; Jin, H.; Zhu, Z.-G.; Zhang, J.-J.; Pang, J.-X.; et al. Praeruptorin A inhibits lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB pathway activation. Phytother. Res. PTR 2011, 25, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.J.; Kim, J.; Bang, O.-S. Pyranocoumarins from Root Extracts of Peucedanum praeruptorum Dunn with Multidrug Resistance Reversal and Anti-Inflammatory Activities. Mol. Basel Switz. 2015, 20, 20967–20978. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shin, E.M.; Choi, R.J.; Jung, Y.H.; Kim, J.; Tosun, A.; Kim, Y.S. Suppression of LPS-induced inflammatory and NF-κB responses by anomalin in RAW 264.7 macrophages. J. Cell. Biochem. 2011, 112, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Tosun, A.; Kim, Y.S. Anti-inflammatory effect of corymbocoumarin from Seseli gummiferum subsp. corymbosum through suppression of NF-κB signaling pathway and induction of HO-1 expression in LPS-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2016, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Shastri, L.A.; Manjunath, G.D.; Kulkarni, M.V. Dual fluorescence and biological evaluation of paracetamol ethers from 4-bromomethylcoumarins. Indian J. Chem. Sect. B 2004, 43B, 2416–2422. [Google Scholar] [CrossRef]
- Bissonnette, E.Y.; Tremblay, G.M.; Turmel, V.; Pirotte, B.; Reboud-Ravaux, M. Coumarinic derivatives show anti-inflammatory effects on alveolar macrophages, but their anti-elastase activity is essential to reduce lung inflammation in vivo. Int. Immunopharmacol. 2009, 9, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Jabbari, A.; Orafaie, A.; Mehri, R.; Zeraatkar, S.; Ahmadi, T.; Alimardani, M.; Sadeghian, H. Synthesis and SAR studies of mono O-prenylated coumarins as potent 15-lipoxygenase inhibitors. Eur. J. Med. Chem. 2012, 57, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Timonen, J.; Vuolteenaho, K.; Leppänen, T.; Nieminen, R.; Moilanen, E.; Aulaskari, P.; Jänis, J. 7-(2-Oxoalkoxy)coumarins: Synthesis and Anti-Inflammatory Activity of a Series of Substituted Coumarins. J. Heterocycl. Chem. 2015, 52, 1286–1295. [Google Scholar] [CrossRef]
- Victor, E.G.; Silveira, P.C.; Possato, J.C.; da Rosa, G.L.; Munari, U.B.; de Souza, C.T.; Pinho, R.A.; da Silva, L.; Streck, E.L.; Paula, M.M. Pulsed ultrasound associated with gold nanoparticle gel reduces oxidative stress parameters and expression of pro-inflammatory molecules in an animal model of muscle injury. J. Nanobiotechnol. 2012, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Shamim, A.; White, L.S.; Bertino, M.F.; Mesaik, M.A.; Soomro, S. The anti-inflammatory properties of Au–scopoletin nanoconjugates. New J. Chem. 2014, 38, 5566–5572. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Gautam, D.R.; Litinas, K.E.; Papamehael, T. Synthesis of some 3,4-dihydro-2H-benzo[f]pyrano[2,3-h]chromen-6-one derivatives. J. Chem. Soc. [Perkin 1] 2002, 1455–1460. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Gautam, D.R.; Litinas, K.E.; Hadjipavlou-Litina, D.J.; Fylaktakidou, K.C. Synthesis and evaluation of the antioxidant and antiinflammatory activities of some benzo[l]khellactone derivatives and analogues. Eur. J. Med. Chem. 2004, 39, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Tsoukka, M.; Litinas, K.E.; Nicolaides, D.N.; Hadjipavlou-Litina, D.J. Synthesis and biological evaluation of new benzo[f]furo[2,3-h]-and benzo[f]pyrano[2,3-h]coumarin derivatives. J. Heterocycl. Chem. 2007, 44, 529–534. [Google Scholar] [CrossRef]

| Cpd | CPE% 1 | IL-1β | IL-6 | 5-LOX | NF-κB | NO | TNF-α | Ref. |
|---|---|---|---|---|---|---|---|---|
| 4 | IC50: 6.6 2 | [5] | ||||||
| 5 | <200 ng/mL (20 mg/kg) 3,** | <400 ng/mL (20 mg/kg) 3,** | [7] | |||||
| 6 | <3000 pg/mL (160 μg/mL, 24 h) 4,*; <1000 pg/mL (160 μg/mL) 5,** | Activity < 1 (160 μM) 4,* | <20 μM (160 μM) 4,** | <500 pg/mL (160 μg/mL, 24 h) 4,*; <400 pg/mL (160 μg/mL) 5,** | [10] | |||
| 14 | 39% (0.01 mmol/kg) | IC50: 72.5 nm | [12] | |||||
| 15 | <10 μM (10 μM) 6,* | [13] | ||||||
| 16 | >80% (50 μg/mL) 1 | [14] | ||||||
| 17 | <10% (50 μg/mL) 1 | [14] | ||||||
| 18 | <5% (50 μg/mL) 7 | [14] | ||||||
| 21 | 0% 6 | 0% 6 | 0% 6 | [18] |
| Cpd | COX-2 | IL-1β | IL-6 | IL-10 | NF-κB | NO | iNO | PGE2 | TNF-α | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | <40% (10 μg/mL) 3,*** | <20 pg/mL (10 μg/mL) 1,*; <300 pg/mL (30 mg/kg) 2,* | <600 pg/mL (30 mg/kg) 2,* | <600 pg/mL (15 mg/kg) 2,** | <30 μM (10 μg/mL) 3,***; <6 μM (10 mg/kg) 4; IC50: 52.3 ± 5.4 μM 3,*** | <40% (10 μg/mL) 3,***; 83.3% (20 μg/mL) 3 | <400 pg/mL (10 μg/mL) 3,***; <80 pg/mL (10 mg/kg) 4,*** | <40 μM (10 ng/mL) 3,***; <300 ng/mL (10 mg/kg) 4; <1500 pg/mL (30 mg/kg) 2,** | [21,22,27,28,34] | |
| 23 | IC50: 38.3 ± 1.7 μM 3,*** | [22] | ||||||||
| 24 | IC50: 10.7 μM 5 | IC50>20 μg/mL 3, 28.1% ± 39.7% (20 μg/mL) 3 | [32,35] | |||||||
| 25 | <20% (20 μg/mL) 3,**** | 92.4% (20 μg/mL) 3 | [34] | |||||||
| 26 | <60% at 50 μM 6,* | IC50: 9.8 μg/mL 3; 85.3% ± 8.7% (20 μg/mL) 3 | <5 μM (20 μg/mL) 3** | <10 ng/mL (50 μM) 6,* | [35,36] |
| Cpd | COX-2 | IL-1β | IL-6 | NF-κB | NO | iNO | PGE2 | TNF-α | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 28 | <20% (30 μM) 1,*** | <40% (30 μM) 1,*** | <20 μM (30 μM) 1,***; <40 μM (30 μM) 2,** | <20% (30 μM) 1,*** | <40% (30 μM) 1,*** | [40] | |||
| 29 | <100 pg/mL (25 μg/mL) 1,** | <100 pg/mL (100 μM) 1,** | <15 μM (25 μg/mL) 1,** | <10000 pg/mL (25 μg/mL) 1,* | [41,42] | ||||
| 30 | <60% (50 μM) 1,*** | <4000 RFU (50 μM) for NF-κB-dependent alkaline phosphate (SEAP ) 1,*** | <60% (50 μM) 1,*** | <60% (50 μM) 1,*** | [43] | ||||
| 31 | <8000 RFU (60 μg/mL) 1,** | <20 μM (60 μM) 1,*** | <500 pg/mL (60 μM) 1,*** | [44] |
| Cpd | COX-2 | CPE% 3 | IL-6 | LOX | NO | iNO | TNF-α | Ref. |
|---|---|---|---|---|---|---|---|---|
| 32, R = 6-Cl | 69 (3 h); 60 (6 h) * | [45] | ||||||
| 35, R1 = 5-chloropyridin-3-yl; R2 = CH3COOCH2 | <600 pg/mL (1 μM/kg) 1,* | <1200 pg/mL (1 μM/kg) 1,* | [46] | |||||
| 38a | −8 ± 17.4 (10 μM) 2; 37 ± 15.2 (100 μM) 2 | IC50: 10 μM 2; 33 ± 4.6 (10 μM) 2; 89 ± 0.7 (100 μM) 2 | IC30: 30 μM 2; 49 ± 2 (10 μM) 2; 90 ± 1.6 (100 μM) 2 | 58 ± 5.5 (10 μM) 2; 99 ± 0.1 (100 μM) 2 | [48] | |||
| 38b | 29 ± 7.8 (10 μM) 2 ; 57 ± 1.6 (100 μM) 2 | IC50: 24 μM 2; 47 ± 4.5 (10 μM) 2; 90 ± 0.4 (100 μM) 2 | IC50: 21 μM 2; 27 ± 3.6 (10 μM) 2; 89 ± 0.4 (100 μM) 2 | 47 ± 7.2 (10 μM) 2; 97 ± 0.6 (100 μM) 2 | [48] | |||
| 39a, R = OH | 48.7 (0.1 mmol/kg, 3.5 h) | 89.8% (1 mM) | [52] | |||||
| 39b, R = H | 54 (0.1 mmol/kg, 3.5 h) | No (1 mM) | [52] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsch, G.; Abdelwahab, A.B.; Chaimbault, P. Natural and Synthetic Coumarins with Effects on Inflammation. Molecules 2016, 21, 1322. https://doi.org/10.3390/molecules21101322
Kirsch G, Abdelwahab AB, Chaimbault P. Natural and Synthetic Coumarins with Effects on Inflammation. Molecules. 2016; 21(10):1322. https://doi.org/10.3390/molecules21101322
Chicago/Turabian StyleKirsch, Gilbert, Ahmed Bakr Abdelwahab, and Patrick Chaimbault. 2016. "Natural and Synthetic Coumarins with Effects on Inflammation" Molecules 21, no. 10: 1322. https://doi.org/10.3390/molecules21101322






