Enantioseparation Using Cellulose Tris(3,5-dimethylphenylcarbamate) as Chiral Stationary Phase for HPLC: Influence of Molecular Weight of Cellulose †
Abstract
:1. Introduction
2. Results and Discussion
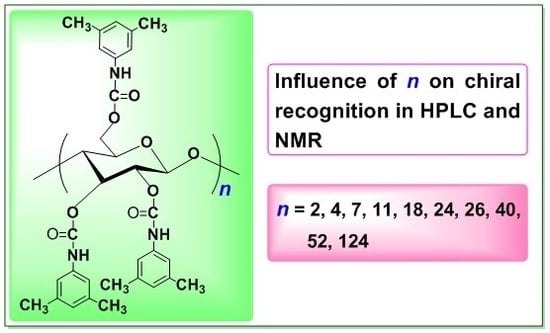
2.1. Structure of CDMPCs
2.2. Chiral Recognition Ability of CDMPCs in HPLC
2.3. Chiral Recognition Ability of CDMPC in NMR
3. Materials and Methods
3.1. Chemicals
3.2. Measurements
3.3. Preparation of Cellulose with Lower DPs
3.4. Synthesis of 3,5-Dimethylphenylcarbamates of Cellulose with Various DPs
3.5. Preparation of CSPs Based on CDMPCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allenmark, S.G. Chromatographic Enantioseparation: Methods and Applications; Ellis Harwood: Chichester, UK, 1988. [Google Scholar]
- Separations and Analysis. In Comprehensive Chirality; Carreira, E.M.; Yamamoto, H. (Eds.) Elsevier: Amsterdam, The Netherlands, 2012; Volume 8.
- Kuang, X.; Ma, Y.; Su, H.; Zhang, J.; Dong, Y.-B.; Tang, B. High-Performance Liquid Chromatographic Enantioseparation of Racemic Drugs Based on Homochiral Matal-Organic Framework. Anal. Chem. 2014, 86, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Ikai, T.; Kanoh, S.; Yashima, E.; Maeda, K. Switchable Enantioseparation Based on Macromolecular Memory of a Helical Polyacetylene in the Solid State. Nat. Chem. 2014, 6, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of Chirality and Macroring in Imprinted Polymers with Enantiodiscriminative Power. ACS Appl. Mater. Interfaces 2015, 7, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Recent Developments on Polysaccharide-Based Chiral Stationary Phases for Liquid-Phase Separation of Enantiomers. J. Chromatogr. A 2012, 1269, 26–51. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ikai, T.; Okamoto, Y. Synthesis and Application of Immobilized Polysaccharide-Based Chiral Stationary Phases for Enantioseparation by High-Performance Liquid Chromatography. J. Chromatogr. A 2014, 1363, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Aburatani, R.; Okamoto, Y.; Hatada, K. Optical Resolving Ability of 3,5-Dimethylphenylcarbamates of Oligosaccharides and Cyclodextrins. Bull. Chem. Soc. Jpn. 1990, 63, 3606–3610. [Google Scholar] [CrossRef]
- Kasuya, N.; Kusaka, Y.; Habu, N.; Ohnishi, A. Development of Chiral Stationary Phases Consisting of Low-molecular-weight Cellulose Derivatives Covalently Bonded to Silica Gel. Cellulose 2002, 9, 263–269. [Google Scholar] [CrossRef]
- Isogai, A.; Usuda, M. Preparation of Low-molecular-weight Cellulose Using Phosphoric Acid. Mokuzai Gakkaishi 1991, 37, 339–344. [Google Scholar]
- Kaida, Y.; Okamoto, Y. Optical Resolution on Regioselectively Carbamoylated Cellulose and Amylose with 3,5-Dimethylphenyl and 3,5-Dichlorophenyl Isocyanates. Bull. Chem. Soc. Jpn. 1993, 66, 2225–2232. [Google Scholar] [CrossRef]
- Yamamoto, C.; Yashima, E.; Okamoto, Y. Computational Studies on Chiral Discrimination Mechanism of Phenylcarbamate Derivatives of Cellulose. Bull. Chem. Soc. Jpn. 1999, 72, 1815–1825. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawashima, M.; Hatada, K. Controlled Chiral Recognition of Cellulose Triphenylcarbamate Derivatives Supported on Silica Gel. J. Chromatogr. 1986, 363, 173–186. [Google Scholar] [CrossRef]
- Koller, M.; Rimböck, K.-H.; Mannschrek, A. High-Pressure Liquid Chromatography on Triacetyl Cellulose: Characterization of a Sorbent For the Separation of Enantiomers. J. Chromatogr. 1983, 282, 89–94. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.






| Cellulose Oligomer | Avicel (g) | 85% H3PO4 +H2O (mL) | Time (Day) | Stirring | H2O b (L) | Yield g (%) | DP c |
|---|---|---|---|---|---|---|---|
| Cel-1 | 10 | 187 + 7.3 | 56 | without | large excess | 0.77 (7.7) | 7.3 |
| Cel-2 d | 20 | 347 + 14.6 | 12 | with | 4.5 | 4.91 (25) | 11.0 |
| Cel-3 | 10 | 187 + 7.3 | 14 | without | 0.6 | 8.14 (81) | 18.0 |
| Cel-4 | 3 | 56 + 2 | 14 | without | 0.7 | 1.47 (49) | 19.1 |
| Cel-5 | 5 | 88.5 + 3.7 | 14 | without | 1.4 | 3.85 (77) | 23.6 |
| Cel-6 | 1 | 18.7 + 0.7 | 3 | without | 0.25 | 0.89 (89) | 40.2 |
| Cel-7 | 1 | 18.7 + 2.6 | 0.7 | with | 0.25 | 0.85 (85) | 51.6 |
| CDMPC- | Cellulose Oligomer | Reaction Solvent a | Reaction Time (h) | Solvent for CDMPC ppt b | Yield (%) | Mn × 10−3 (Mw/Mn) | DP SEC | DP NMR |
|---|---|---|---|---|---|---|---|---|
| 2 c | Cellobiose | pyridine | MeOH–H2O (4:1) | |||||
| 4 c | Cellotetraose | pyridine | MeOH–H2O (4:1) | |||||
| 7 | Cel-1 | pyridine | 20 | MeOH–H2O (4:1) | 30 | 4.4 (1.13) | 7.3 | 7 |
| 11 | Cel-2 | DMA-Li-py | 20 | MeOH | 55 | 6.7 (1.36) | 11.0 | |
| 18 | Cel-3 | DMA-Li-py | 24 | MeOH–H2O (9:1) | 99 | 10.8 (1.71) | 18.0 | |
| 19 | Cel-4 | pyridine | 41 | MeOH | 18 | 11.5 (1.36) | 19.1 | |
| 24 | Cel-3 | pyridine | 48 | MeOH–H2O (9:1) | 89 | 14.2 (1.86) | 23.6 | 19 |
| 26 | Cel-5 | pyridine | 20 | MeOH | 55 | 15.5 (1.40) | 25.7 | 29 |
| 40 | Cel-6 | pyridine | 72 | MeOH | 89 | 24.2 (1.60) | 40.2 | |
| 52 | Cel-7 | pyridine | 17 d | MeOH | 85 | 31.1 (1.65) | 51.6 | |
| 124 | Avicel | pyridine | 20 | MeOH | 82 | 74.7 (2.90) | 124 |
| CSP= | CDMPC-2 b | CDMPC-4 b | CDMPC-7 | CDMPC-11 | CDMPC-18 | CDMPC-24 | CDMPC-26 | CDMPC-40 d | CDMPC-52 d | CDMPC-124 | CDMPC-124 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eluent c= | H/I = 98/2 | H = 100 | H/I = 99/1 | H/I = 99/1 | H/I = 99/1 | H/I = 99/1 | H/I = 99/1 | H/I = 99/1 | H/I = 99/1 | H/I = 99/1 | H/I = 90/10 | |||||||||||
| Racemate | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α | k1′ | α |
| 1 | 0.26 (–) | ~1 | 1.54 (+) | 1.21 | 1.33 (+) | 1.08 | 1.44 (+) | 1.33 | 1.49 (+) | 1.38 | 2.19 (+) | 1.27 | 1.81 (+) | 1.27 | 2.21 (+) | 1.29 | 2.49 (+) | 1.31 | 1.85 (+) | 1.30 | 0.74 (+) | 1.50 |
| 2 | 0.25 (–) | ~1 | 2.13 (–) | 1.13 | 1.50 (–) | 1.15 | 2.31 (–) | 1.29 | 1.57 (–) | 1.40 | 2.56 (–) | 1.17 | 2.14 (–) | 1.22 | 2.47 (–) | 1.18 | 3.03 (–) | 1.20 | 2.33 (–) | 1.22 | 0.91 (–) | 1.29 |
| 3 | 5.25 (+) | 1.16 | 8.22 (+) | 1.16 | 5.29 (+) | 1.42 | 7.28 (+) | 1.43 | 7.53 (+) | 1.42 | 1.37 (+) | 1.67 | 8.24 (+) | 1.57 | 6.82 (+) | 1.54 | 2.06 (+) | 1.40 | ||||
| 4 | 1.72 | 1.00 | 5.48 (–) | 2.05 | 11.9 (–) | 1.71 | 4.66 (–) | 2.94 | 26.9 (–) | 2.41 | 15.9 (–) | 2.21 | 33.2 (–) | 2.34 | 36.6 (–) | 3.04 | 22.4 (–) | 3.19 | 1.54 (–) | 2.60 | ||
| 5 | 3.11 (+) | 1.15 | 4.03 (+) | 1.17 | 6.67 (+) | 1.06 | 5.14 (+) | 1.09 | 5.82 (+) | 1.10 | 1.23 (+) | 1.23 | ||||||||||
| 6 | 2.58 | 1.00 | 2.33 | 1.00 | 1.31 (+) | ~1 | 2.00 (+) | 1.07 | 2.18 (+) | ~1 | 2.15 (–) | 1.12 | 1.72 (–) | 1.13 | 1.26 (+) | 1.14 | 0.34 (+) | 1.00 | ||||
| 7 | 1.38 (+) | 1.37 | 9.38 | 1.00 | 0.64 (–) | 1.13 | 0.83 (+) | 1.17 | 0.91 (–) | 2.32 | 1.25 (–) | 2.55 | 1.05 (–) | 2.21 | 1.14 (–) | 2.38 | 1.53 (–) | 2.75 | 1.21 (–) | 2.82 | 0.60 (–) | 1.95 |
| 8 | 0.29 (+) | 1.38 | 3.70 (+) | ~1 | 2.22 (–) | ~1 | 3.17 | 1.00 | 1.23 (–) | 1.23 | 3.42 (–) | 1.25 | 2.86 (–) | 1.25 | 3.21 (–) | 1.23 | 4.00 (–) | 1.30 | 3.00 (–) | 1.36 | 1.14 (–) | 1.32 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, Y.; Yamamoto, C.; Kamigaito, M.; Gao, Y.; Shen, J.; Okamoto, Y. Enantioseparation Using Cellulose Tris(3,5-dimethylphenylcarbamate) as Chiral Stationary Phase for HPLC: Influence of Molecular Weight of Cellulose. Molecules 2016, 21, 1484. https://doi.org/10.3390/molecules21111484
Okada Y, Yamamoto C, Kamigaito M, Gao Y, Shen J, Okamoto Y. Enantioseparation Using Cellulose Tris(3,5-dimethylphenylcarbamate) as Chiral Stationary Phase for HPLC: Influence of Molecular Weight of Cellulose. Molecules. 2016; 21(11):1484. https://doi.org/10.3390/molecules21111484
Chicago/Turabian StyleOkada, Yuji, Chiyo Yamamoto, Masami Kamigaito, Yuan Gao, Jun Shen, and Yoshio Okamoto. 2016. "Enantioseparation Using Cellulose Tris(3,5-dimethylphenylcarbamate) as Chiral Stationary Phase for HPLC: Influence of Molecular Weight of Cellulose" Molecules 21, no. 11: 1484. https://doi.org/10.3390/molecules21111484