Inflammasomes and Natural Ingredients towards New Anti-Inflammatory Agents
Abstract
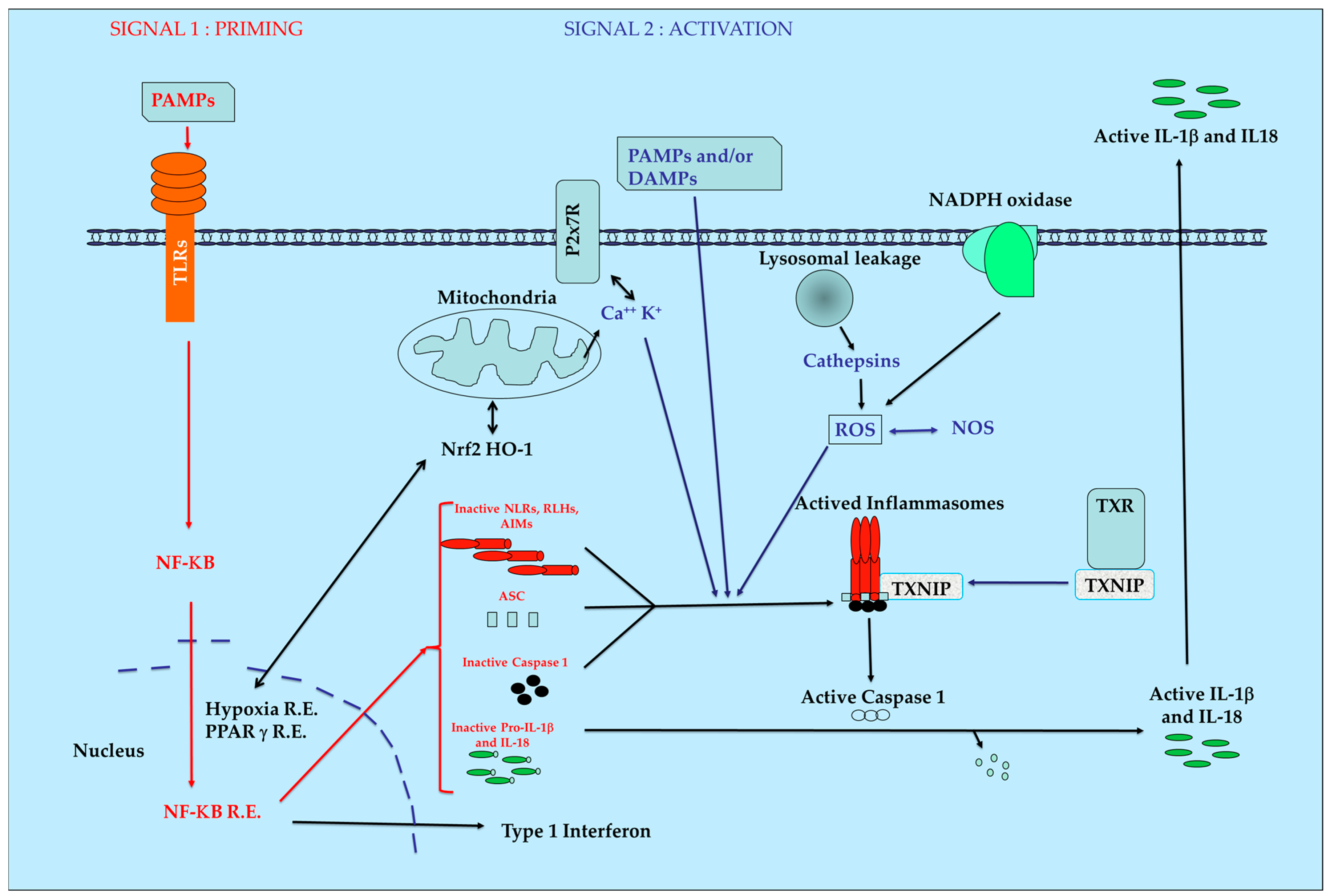
:1. Introduction
2. PRR Families
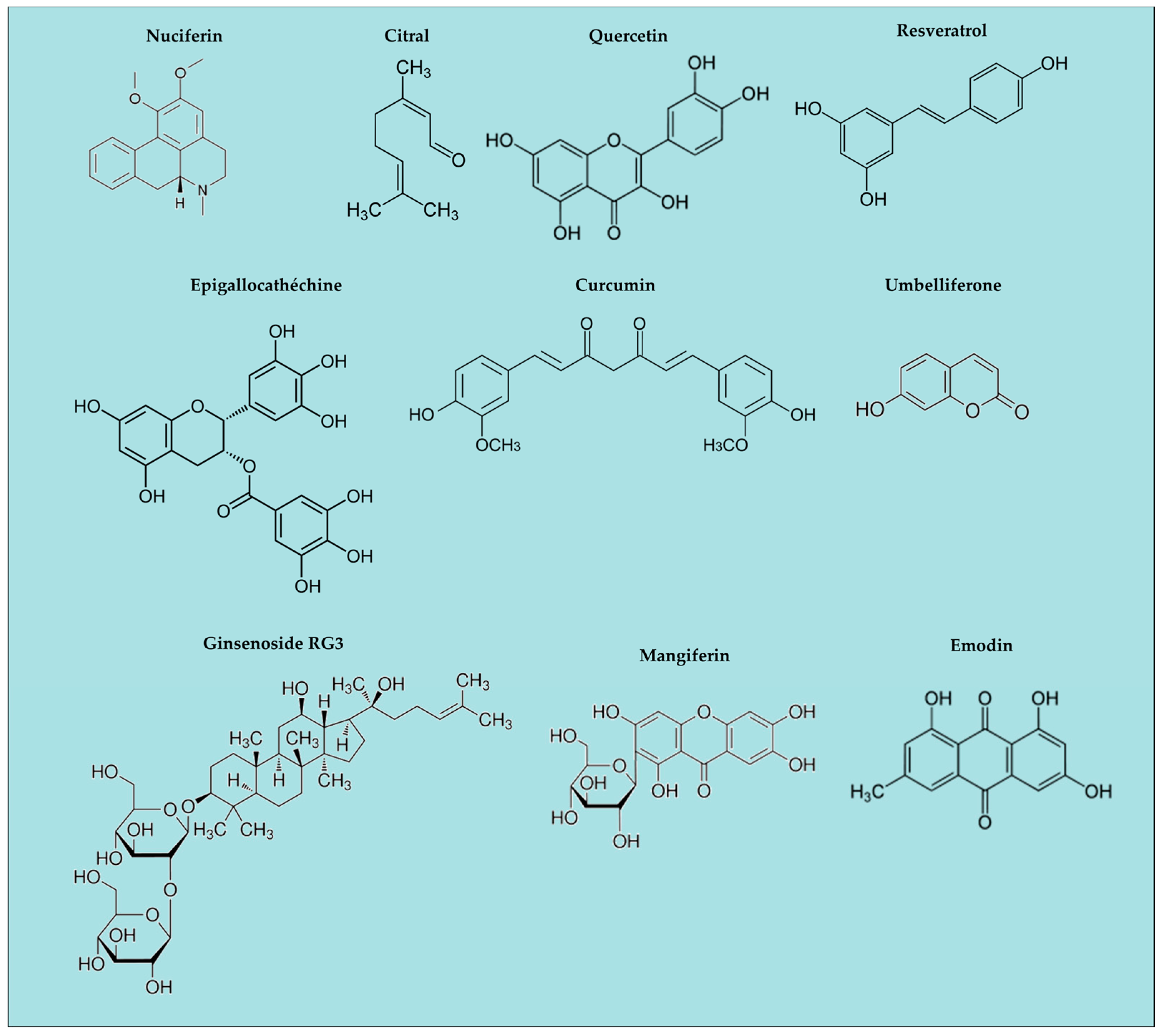
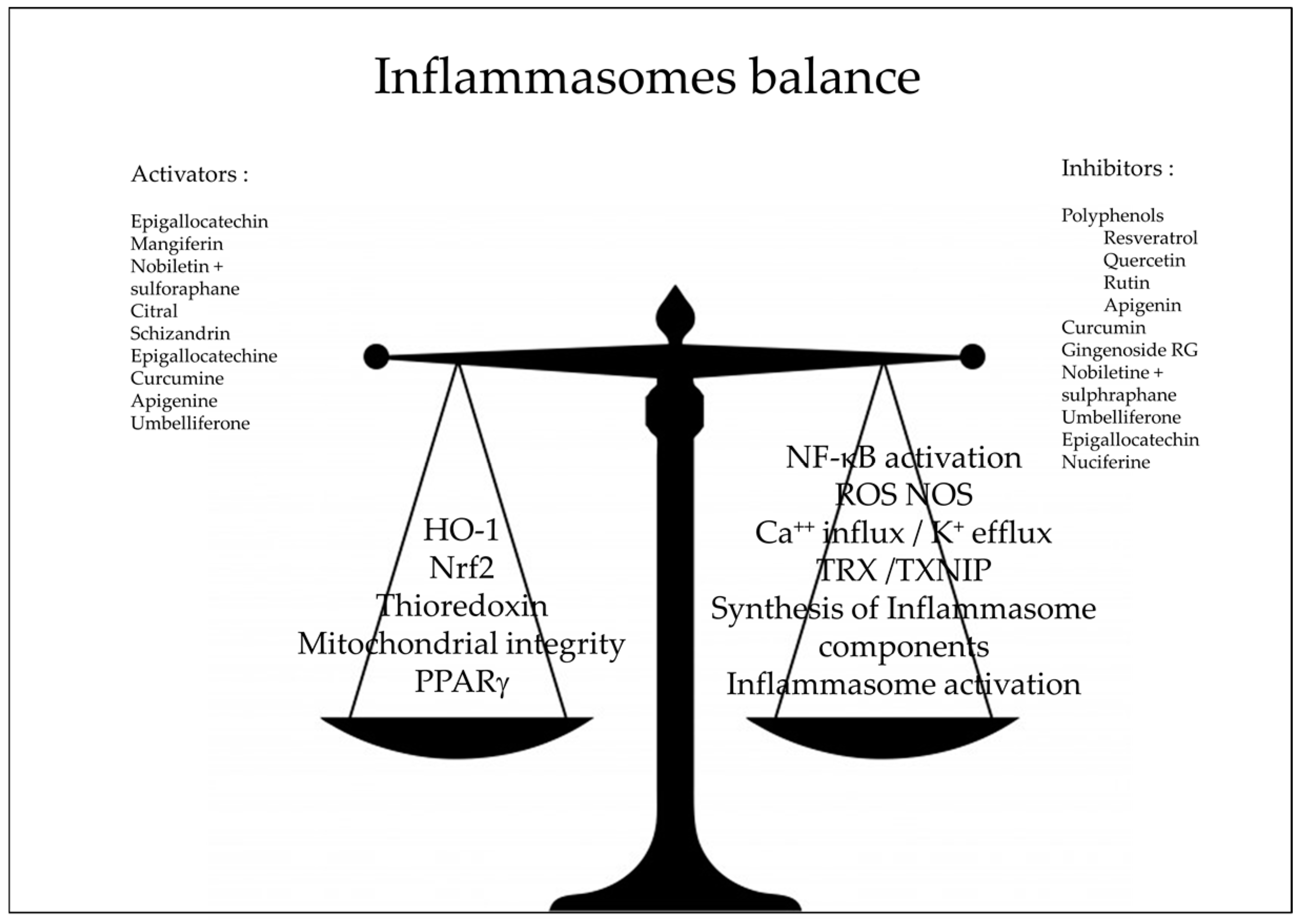
3. Natural Anti-Oxidants and Inflammasomes
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. J. Mol. Cell. 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Shi, L.; Wang, Y.; Chen, S.; Zhang, J. Recent Advances of the NLRP3 Inflammasome in Central Nervous System Disorders. J. Immunol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.M.; Broderick, L. The role of the inflammasome in patients with autoinflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lénárt, N.; Brough, D.; Dénes, Á. Inflammasomes link vascular disease with neuroinflammation and brain disorders. J. Cereb. Blood Flow Metab. 2016, 36, 1668–1685. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 2016, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Dong, Q.; Song, Z.; Shen, F.; Shi, J.; Li, Y. NLRP3 inflammasome: A promising target in ischemic stroke. Inflamm. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Conley, S.M.; Abais, J.M.; Boini, K.M.; Li, P.L. Inflammasome Activation in Chronic Glomerular Diseases. Curr. Drug Targets 2016. [Google Scholar] [CrossRef]
- Hu, Z.; Chai, J. Structural Mechanisms in NLR Inflammasome Assembly and Signaling. Curr. Top. Microbiol. Immunol. 2016, 397, 23–42. [Google Scholar] [PubMed]
- Sharma, N.; Jha, S. NLR-regulated pathways in cancer: Opportunities and obstacles for therapeutic interventions. Cell. Mol. Life Sci. 2016, 73, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Mori, K.; Corradini, N.; Redini, F.; Heymann, D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expert Opin. Pharmacother. 2011, 12, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardin, A.; Lefebvre, M.L.; Labroquère, K.; Faure, O.; Abastado, J.P. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr. Cancer Drug Targets 2006, 6, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.W. Anti-inflammatory glucocorticoid drugs: Reflections after 60 years. Inflammopharmacology 2011, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Ichim, G.; Green, D. Die another way—Non-apoptotic mechanisms of cell death. J. Cell Sci. 2014, 127, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Esmon, C.T. Bench-to-bedside review: Functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit. Care 2003, 7, 23–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliman, H.B.; Piantadosi, C.A. Mitochondrial Quality Control as a Therapeutic Target. Pharmacol. Rev. 2016, 68, 20–48. [Google Scholar] [CrossRef] [PubMed]
- Barančík, M.; Grešová, L.; Barteková, M.; Dovinová, I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 2016, 65 (Suppl. 1), S1–S10. [Google Scholar] [PubMed]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, K.; Vodovotz, Y.; Azhar, N.; Barclay, D.; Janjic, J.M.; Pollock, J.A. In vivo and systems biology studies implicate IL-18 as a central mediator in chronic pain. J. Neuroimmunol. 2015, 283, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Martinon, F. Method for the Identification of Compounds Susceptible to Inhibit Inflammation. EP1746167, 24 January 2007. [Google Scholar]
- Dixit, V.D. Compositions and Methods for Treating NLRP3 Inflammasome-Related Diseases and Disorders. WO2016123229, 4 August 2016. [Google Scholar]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Kim, I.C.; Han, S.J.; Jo, D.G. Composition for Preventing or Treating Neurodegenerative Diseases, Containing Ramalin. WO2016064009, 27 October 2011. [Google Scholar]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, J.H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Hwan, H.U.; Hoon, L.J.; Sung, S.H.; Jae, K.Y. Screening Methods of Preventing or Treating Agent of Inflammation Using Sophora flavescens Extract. KR101602476, 2014. [Google Scholar]
- Piao, X.L.; Piao, X.S.; Kim, S.W.; Park, J.H.; Kim, H.Y.; Cai, S.Q. Identification and Characterization of Antioxidants from Sophora flavescens. Biol. Pharm. Bull. 2006, 29, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Jae, L.M.; Sik, L.G. Composition for Preventing and Treating Inflammatory Disease Comprising Garlic Extract as an Active Ingredient. KR20160023239, 2014. [Google Scholar]
- Koh, Y.S.; Yoo, E.S.; Hyun, J.W.; Kang, H.K.; Lee, N.H.; Suh, I.S. Composition for Preventing and Treating Inflammatory Diseases and Immune Diseases, Containing apo-9′-Fucoxanthinone As Active Ingredient. US2015182487, 2 July 2015. [Google Scholar]
- Han, S.C.; Kang, N.J.; Yoon, W.J.; Kim, S.; Na, M.C.; Koh, Y.S.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; Kang, H.K.; et al. External Application of Apo-9′-fucoxanthinone, isolated from Sargassum muticum, suppresses Inflammatory Responses in a Mouse Model of Atopic Dermatitis. Toxicol. Res. 2011, 32, 109–114. [Google Scholar] [CrossRef]
- Artlett, C.M.; Katsikis, P.D. Methods for Treating or Preventing Fibrosis in Subjects Afflicted with Scleroderma. U.S. 2014314746 (A1), 23 October 2014. [Google Scholar]
- Takatsu, K.; Hirai, Y.; Nagai, Y.; Honda, H.; Matsunaga, T. Inflammasome Activity Control Agent. JP2014094917, 2012. [Google Scholar]
- González-Reyes, S.; Santillán-Cigales, J.J.; Jiménez-Osorio, A.S.; Pedraza-Chaverri, J.; Guevara-Guzmán, R. Glycyrrhizin ameliorates oxidative stress and inflammation in hippocampus and olfactory bulb in lithium/pilocarpine-induced status epilepticus in rats. Epilepsy Res. 2016, 126, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ka, S.M.; Lin, J.C.; Lin, T.J.; Liu, F.C.; Chao, L.K.; Ho, C.L.; Yeh, L.T.; Sytwu, H.K.; Hua, K.F.; Chen, A. Citral alleviates an accelerated and severe lupus nephritis model by inhibiting the activation signal of NLRP3 inflammasome and enhancing Nrf2 activation. Arthritis Res. Ther. 2015, 17, 331. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, X.; Liu, Z.; Meng, R.; Chen, X.; Guo, N. Antimicrobial, antioxidant, and antitumor activity of epsilon-poly-L-lysine and citral, alone or in combination. Food Nutr. Res. 2016, 15, 31891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhao, B.L.; Liu, G.T.; Xin, W.J. Scavenging effects on active oxygen radicals by schizandrins with different structures and configurations. Free Radic. Biol. Med. 1990, 9, 99–104. [Google Scholar] [PubMed]
- Leong, P.K.; Ko, K.M. Schisandrin B induces an Nrf2-mediated thioredoxin expression and suppresses the activation of inflammasome in vitro and in vivo. Biofactors 2015, 41, 314–323. [Google Scholar] [PubMed]
- Chun, J.N.; Cho, M.; So, I.; Jeon, J.H. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: A review of the molecular mechanisms. Fitoterapia 2014, 97, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.H.; Chen, X.Y.; Hu, Q.H.; Wang, M.X.; Jin, R.; Zhang, Q.Y.; Wang, W.; Wang, R.; Kang, L.L.; et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid. Redox Signal. 2015, 22, 848–870. [Google Scholar] [CrossRef] [PubMed]
- Aruna, R.; Geetha, A.; Suguna, P. Rutin modulates ASC expression in NLRP3 inflammasome: A study in alcohol and cerulein-induced rat model of pancreatitis. Mol. Cell. Biochem. 2014, 396, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Zhang, X.; Pan, Y.; Li, Y.C.; Kong, L.D. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem. Pharmacol. 2012, 84, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Lai, H.C.; Chen, Y.B.; Chen, L.G.; Wu, Y.H.; Ko, Y.F.; Lu, C.C.; Chang, C.J.; Wu, C.Y.; Martel, J.; et al. cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. Innate Immun. 2014, 20, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Saitoh, T.; Kozaki, T.; Park, S.; Takahama, M.; Akira, S. Resveratrol inhibits the acetylated α-tubulin-mediated assembly of the NLRP3-inflammasome. Int. Immunol. 2015, 27, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, R.; Yang, J.; Yang, J.; Ding, J.; Wu, H.; Zhang, J. Resveratrol pretreatment protects rat hearts from ischemia/reperfusion injury partly via a NALP3 inflammasome pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8731–8741. [Google Scholar] [PubMed]
- Yang, S.J.; Lim, Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism 2014, 63, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Qin, T.; Qu, R.; Ma, S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016, 296, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Blanquicett, C.; Kang, B.Y.; Ritzenthaler, J.D.; Jones, D.P.; Hart, C.M. Oxidative stress modulates PPAR gamma in vascular endothelial cells. Free Radic. Biol. Med. 2010, 48, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Miller, J.M.; Thompson, J.K.; MacPherson, M.B.; Beuschel, S.L.; Westbom, C.M.; Sayan, M.; Shukla, A. Curcumin: A double hit on malignant mesothelioma. Cancer Prev. Res. (Phila) 2014, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, Y.; Hu, Y.; Wu, X.; Wang, Y.; Zhang, X.; Fu, J.; Zou, X.; Zhang, J.; Chen, X.; et al. Targeting HO-1 by Epigallocatechin-3-Gallate Reduces Contrast-Induced Renal Injury via Anti-Oxidative Stress and Anti-Inflammation Pathways. PLoS ONE 2016, 11, e0149032. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Seo, G.; Lee, J.Y.; Chae, G.T.; Lee, S.B. Epigallocatechin-3-gallate attenuates the AIM2-induced secretion of IL-1β in human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2015, 467, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014, 745, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Doss, H.M.; Dey, C.; Sudandiradoss, C.; Rasool, M.K. Targeting inflammatory mediators with ferulic acid, a dietary polyphenol, for the suppression of monosodium urate crystal-induced inflammation in rats. Life Sci. 2016, 148, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on Pharmacological properties of Coumarins. Mini Rev. Med. Chem. 2016, 16. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.; Wang, X.; Fu, Q.; Ma, S. Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 2015, 600, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Malterud, K.E.; Farbrot, T.L.; Huse, A.E.; Sund, R.B. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology 1993, 47 (Suppl. 1), 77–85. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Shim, D.W.; Shin, W.Y.; Heo, K.H.; Kwak, S.B.; Sim, E.J.; Jeong, J.H.; Kang, T.B.; Lee, K.H. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation. Int. J. Mol. Sci. 2015, 16, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sadhukhan, P.; Sil, P.C. Mangiferin: A xanthonoid with multipotent anti-inflammatory potential. Biofactors 2016. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Harashima, M.; Kurogi, K.; Suiko, M.; Liu, M.C.; Sakakibara, Y. A novel procedure for the assessment of the antioxidant capacity of food components. Anal. Biochem. 2016, 507, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, H.; Song, C.; Zhang, F.; Liu, Y.; Wu, J.; Wen, X.; Liang, C.; Ma, K.; Li, L.; et al. Mangiferin regulates cognitive deficits and heme oxygenase-1 induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2015, 29, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rashid, K.; Sadhukhan, P.; Agarwal, N.; Sil, P.C. Attenuative role of mangiferin in oxidative stress-mediated liver dysfunction in arsenic-intoxicated murines. Biofactors. 2016, 42, 515–532. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Peng, X.; Zhu, J.; Chen, X.; Liu, H.; Tang, C.; Dong, Z.; Liu, F.; Peng, Y. Mangiferin attenuate sepsis-induced acute kidney injury via antioxidant and anti-inflammatory effects. Am. J. Nephrol. 2014, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism 2015, 64, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.W.; Pan, Z.Z.; Hu, J.J.; Chen, W.L.; Zhou, G.Y.; Lin, W.; Jin, L.X.; Xu, C.L. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur. J. Pharmacol. 2016, 770, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Wang, S.N.; Yang, X.H.; Lan, W.J.; Chen, Z.W.; Chen, J.K.; Xie, J.H.; Han, Y.F.; Pi, R.B.; Yang, X.B. Gartanin Protects Neurons against Glutamate-Induced Cell Death in HT22 Cells: Independence of Nrf-2 but Involvement of HO-1 and AMPK. Neurochem. Res. 2016, 41, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia 2012, 83, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Park, J.Y.; Choi, S.; Lee, J.B.; Jung, H.; Kim, T.D.; Yoon, S.R.; Choi, I.; Shim, S.; Park, Y.J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem. Biophys. Res. Commun. 2015, 463, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, L.; Sehirli, O.; Cetinel, S.; Cikler, E.; Gedik, N.; Sener, G. Protective effect of aqueous garlic extract against renal ischemia/reperfusion injury in rats. J. Med. Food 2005, 8, 319–326. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, S.M.; Binda, N.S.; Nogueira-Machado, J.A.; Vieira-Filho, S.A.; Caligiorne, R.B. The antioxidant properties of organosulfur compounds (sulforaphane). Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V.; Jeffery, E.H. The isothiocyanate erucin induces reactive oxygen species and a transient decrease in glutathione in human liver cancer cells. FASEB J. 2006, 20, A155. [Google Scholar]
- Lee, J.; Ahn, H.; Hong, E.J.; An, B.S.; Jeung, E.B.; Lee, G.S. Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell Immunol. 2016, 306–307, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Gautam, L.N.; Adhikari, D.; Karki, R. A Comprehensive Review on Chemical Profiling of Nelumbo Nucifera: Potential for Drug Development. Phytother. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Liu, Y.L.; Yang, Y.; Zhang, D.M.; Kong, L.D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 2015, 747, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Kao, C.L.; Wu, H.M.; Li, W.J.; Huang, C.T.; Li, H.T.; Chen, C.Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qiu, P.; Xu, G.; Wu, X.; Dong, P.; Yang, G.; Zheng, J.; McClements, D.J.; Xiao, H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J. Agric. Food Chem. 2012, 60, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- López-Castejón, G.; Pelegrín, P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin. Investig. Drugs 2012, 21, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.; Bhagwat, S. Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods; U.S. Department of Agriculture (USDA), Nutrient Data Laboratory: Beltsville, MD, USA, 2010; Release 2.
- Prost, M. Method for Determining the Antiradical Defence Potential and Use Thereof, in Particular in Veterinary and Human Preventive Therapeutics. WO2005040835, 27 October 2005. [Google Scholar]
| Name | Localization | Ligands | Actions |
|---|---|---|---|
| TLR (1 to 13 1) | Trans membrane (cell, endosome) | Pathogen fragments | NF-κB activation Production of immunomodulatory cytokines and chemokines |
| CLRs | Trans membrane or soluble | Carbohydrates | |
| NLRs (NODs) | Intracytoplasmic | Peptidoglycans | Production of IL-1β IL-18 Pyroptosis |
| RLHs | Intracytoplasmic | Ribonucleic acids | |
| AIMs | Intracytoplasmic | Deoxyribonucleic acids |
| Composition | Biological Demonstration | Pathology Target | Reference |
|---|---|---|---|
| β hydroxybutyrate | Bone marrow derived macrophages | NLRp3 related pathologies | [25,26] |
| Ramalin | In vivo behaviour | Neurodegenerative diseases | [27,28] |
| Sophora falvescens extracts | THP-1 inflammation | Acute inflammation | [29,30] |
| Garlic extracts | Cell culture | Inflammation influenza | [31] |
| Fucoxanthinone | Cell culture | Inflammation immune disease | [32,33] |
| Mixture of drugs | Cell culture | Scleroderma | [34] |
| Glycyrrhizin and derivatives | Cell culture | Inflammation auto-immune diseases | [35,36] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutartre, P. Inflammasomes and Natural Ingredients towards New Anti-Inflammatory Agents. Molecules 2016, 21, 1492. https://doi.org/10.3390/molecules21111492
Dutartre P. Inflammasomes and Natural Ingredients towards New Anti-Inflammatory Agents. Molecules. 2016; 21(11):1492. https://doi.org/10.3390/molecules21111492
Chicago/Turabian StyleDutartre, Patrick. 2016. "Inflammasomes and Natural Ingredients towards New Anti-Inflammatory Agents" Molecules 21, no. 11: 1492. https://doi.org/10.3390/molecules21111492






