Piper sarmentosum Effects on 11β-Hydroxysteroid Dehydrogenase Type 1 Enzyme in Serum and Bone in Rat Model of Glucocorticoid-Induced Osteoporosis
Abstract
:1. Introduction
2. Results
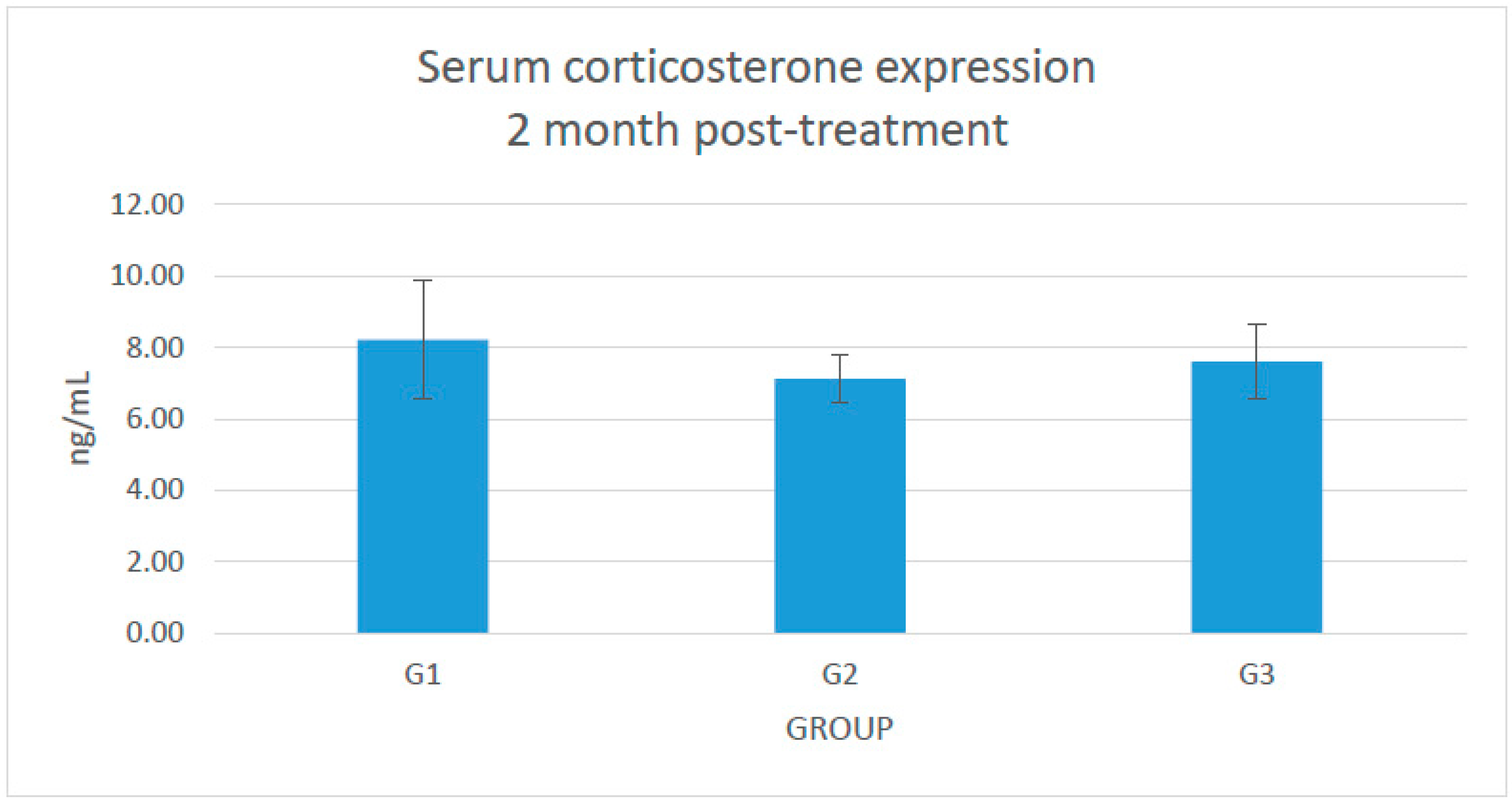
2.1. Changes in the Serum Level of Corticosterone
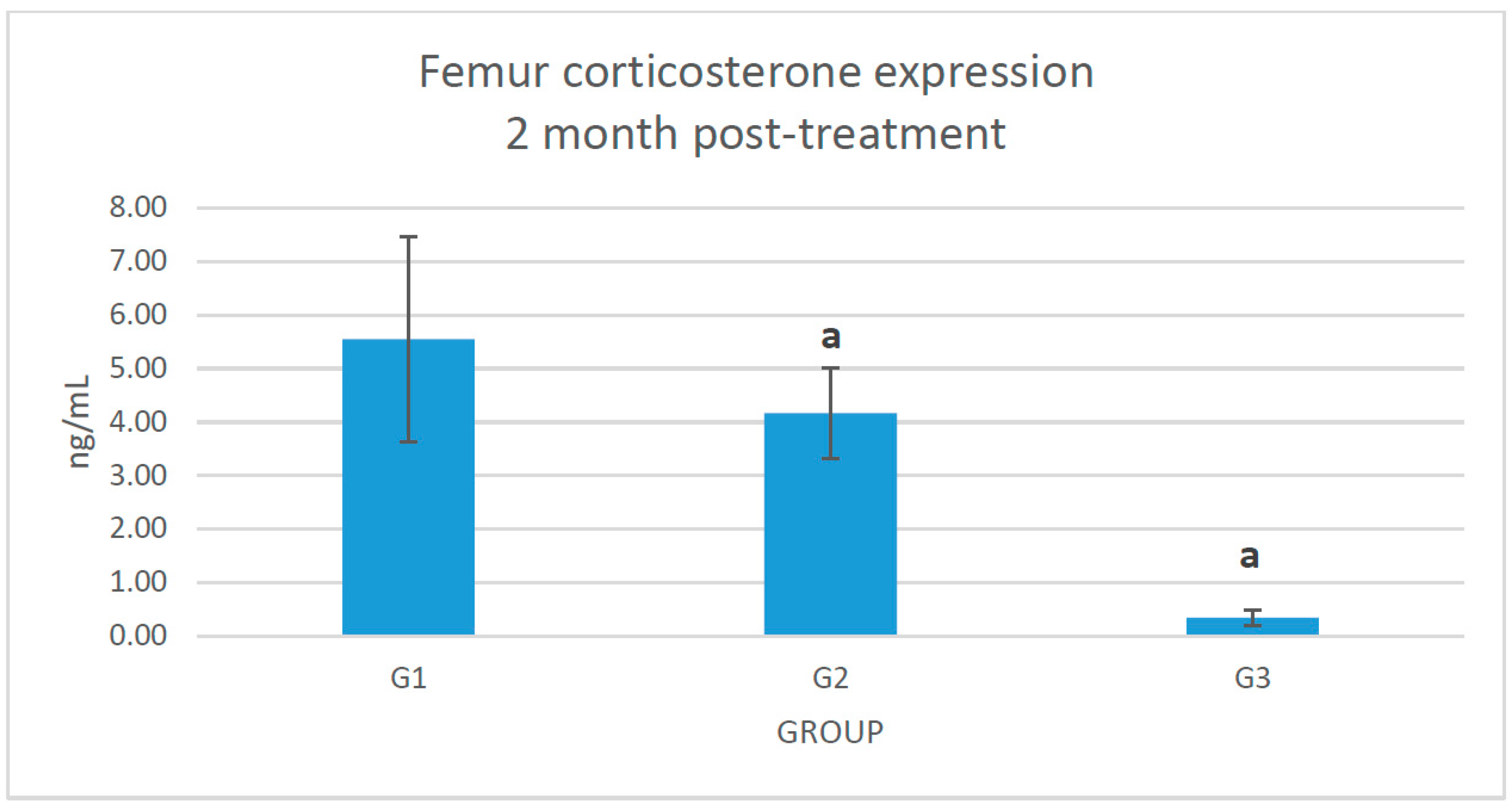
2.2. Changes of Corticosterone Level in the Femoral Bone
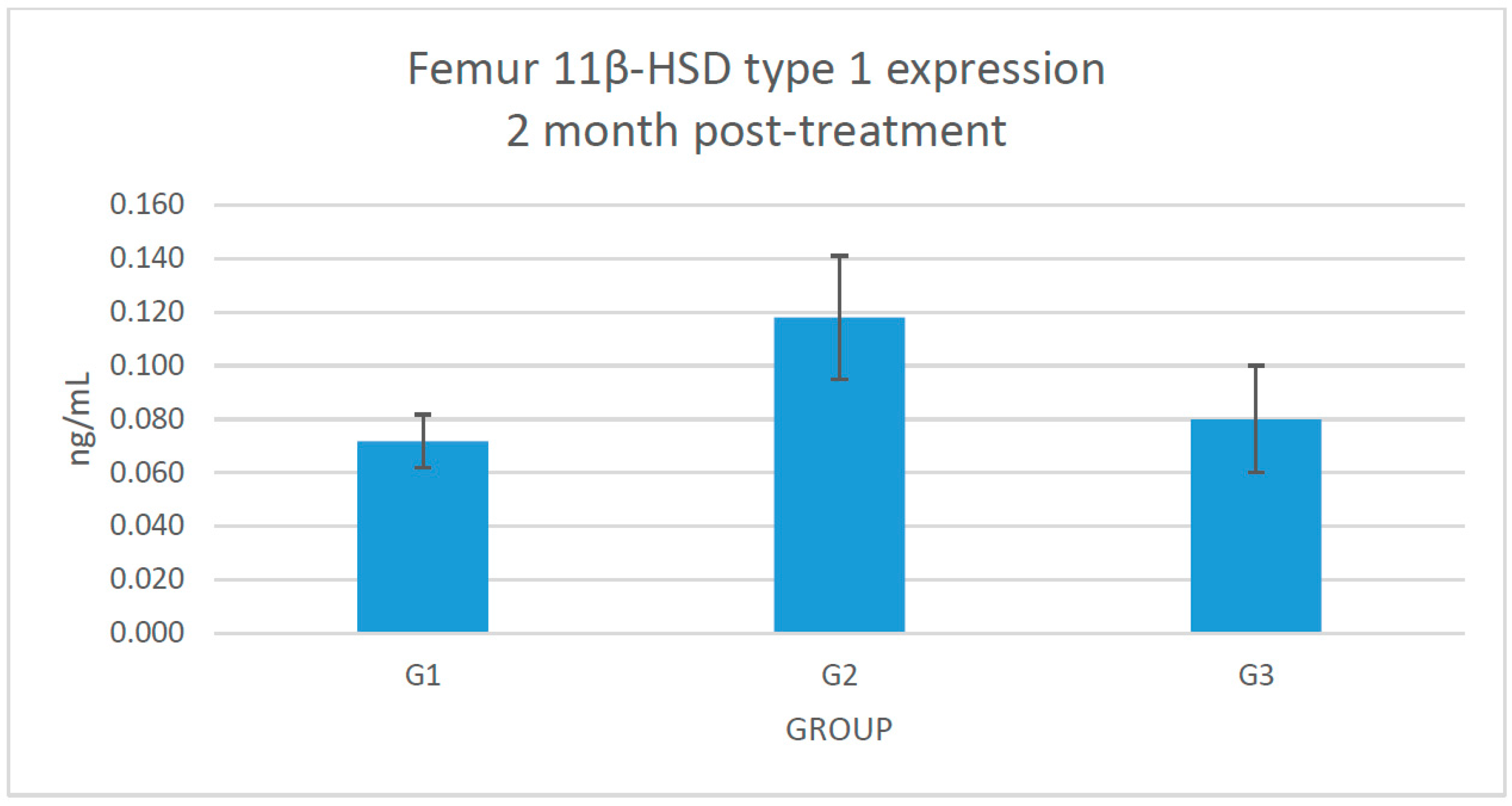
2.3. Changes of 11β-HSD Type 1 Protein Expression in the Femoral Bone
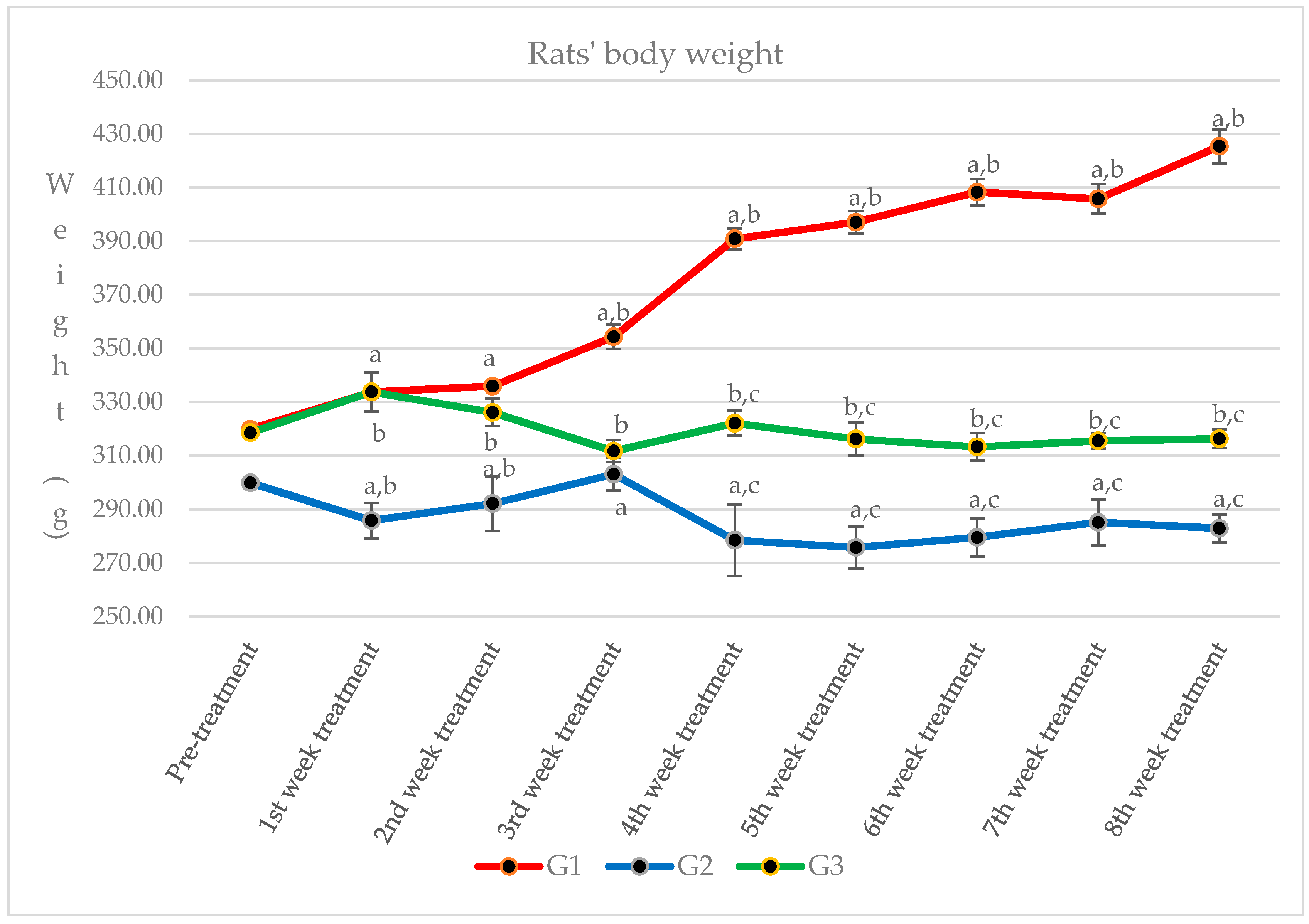
2.4. Changes of Rats’ Body Weight
3. Discussion
4. Materials and Methods
4.1. Preparation of Piper sarmentosum Water Extract
4.2. Animals and Treatment
4.3. Sample Collection
4.4. Measurement of Parameters
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orcel, P. Prevention and treatment of glucocorticoid-induced osteoporosis in 2005. Jt. Bone Spine 2005, 72, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Bilezikian, J.P.; Angeli, A.; Giustina, A. Perspectives on glucocorticoid-induced osteoporosis. Bone 2004, 34, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Zhang, B.; Cooper, C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000, 15, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.; Stewart, P.M. 11β-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Krozowski, Z.S. 11β-hydroxysteroid dehydrogenase. Vitam. Horm. 1997, 57, 249–324. [Google Scholar]
- Eijken, M.; Hewison, M.; Cooper, M.S.; de Jong, F.H.; Chiba, H.; Stewart, P.M.; Uitterlinden, A.G.; Pols, H.A.P.; van Leeuwen, J.P.T.M. 11β-Hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol. Endocrinol. 2005, 19, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Tanaka, A.; Hino, K.; Takamura, H.; Hirano, T.; Oka, K.; Kanazawa, M.; Miwa, T.; Notoya, Y.; Niitsuma, T.; et al. Assessing systemic 11-hydroxysteroid dehydrogenase with serum cortisone/cortisol ratios in healthy subjects and patients with diabetes mellitus and chronic renal failure. Metabolism 2001, 50, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.S.; Walker, E.A.; Bland, R.; Fraser, W.D.; Hewison, M.; Stewart, P.M. Expression and functional consequences of 11β-hydroxysteroid dehydrogenase activity in human bones. Bone 2000, 27, 375–381. [Google Scholar] [CrossRef]
- Edwards, C.R.W.; Burt, D.; McIntyre, M.A.; de Kloet, E.R.; Stewart, P.M.; Brett, L.; Sutanto, W.S.; Monder, C. Localisation of 11β-hydroxysteroid dehydrogenase—Tissue specific protector of the mineralocorticoid receptor. Lancet 1988, 332, 986–989. [Google Scholar] [CrossRef]
- Woitge, H.W.; Harrison, J.R.; Ivkosic, A.; Krozowski, Z.; Kream, B.E. Cloning and in vitro characterization of α1(l)-collagen 11β-hydroxysteroid dehydrogenase type 2 transgene as models for osteoblast-selective inactivation of natural glucocorticoids. Endocrinology 2001, 142, 1341–1348. [Google Scholar] [PubMed]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef] [PubMed]
- Ramli, E.S.M.; Suhaimi, F.; Ahmad, F.; Shuid, A.N.; Mohamad, N.; Ima-Nirwana, S. Piper Sarmentosum: A New Hope for the Treatment of Osteoporosis. Curr. Drug. Targets 2013, 14, 1675–1682. [Google Scholar] [CrossRef]
- Zhou, D.A.; Zheng, H.X.; Wang, C.W.; Shi, D.; Li, J.J. Influence of glucocorticoids on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. BMC Musculoskelet. Disord. 2014, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.; Adenan, M.I.; Ahmad, A.R.; Sahdan, R. Natural antioxidants: Piper sarmentosum (kadok) and Morinda elliptica (mengkudu). Malays. J. Nutr. 2003, 9, 41–51. [Google Scholar] [PubMed]
- Hussain, K.; Ismail, Z.; Sadikun, A.; Ibrahim, P. Analysis of proteins, polysaccharides, glycosaponins contents of Piper sarmentosum Roxb, an anti-TB evaluation for bio-enhancing/interaction effects of leaf extracts with Isoniazid (INH). Nat. Product. Radiance 2008, 7, 402–408. [Google Scholar]
- Ariffin, S.H.Z.; Omar, W.H.H.W.; Ariffin, Z.Z.; Safian, M.F.; Senafi, S.; Wahab, R.M.A. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell Int. 2009, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Peungvicha, P.; Thirawarapan, S.S.; Temsiririrkkul, R.; Watanabe, H.; Prasain, J.K.; Kadota, S. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J. Ethnopharmacol. 1998, 60, 27–32. [Google Scholar] [CrossRef]
- Rahman, N.N.N.A.; Furuta, T.; Kojima, S.; Takane, K.; Mohd, M.A. Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 1999, 64, 249–254. [Google Scholar] [CrossRef]
- Ridtitid, W.; Rattanaprom, W.; Thaina, P.; Chittrakarn, S.; Sunbhanich, M. Neuromuscular blocking activity of methanolic extract of Piper sarmentosum leaves in the rat phrenic nerve-hemidiaphragm preparation. J. Ethnopharmacol. 1998, 61, 135–142. [Google Scholar] [CrossRef]
- Amran, A.A.; Zakaria, Z.; Othman, F.; Das, S.; Raj, S.; Nordin, N.A.M.M. Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis. 2010, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Patahuddin, H.; Mohamad, A.S.; Israf, D.A.; Sulaiman, M.R. In vivo anti-nociceptive and anti-inflammatory activities of the aqueous extract of the leaves of Piper sarmentosum. J. Ethnopharmacol. 2010, 128, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Estai, M.A.; Suhaimi, F.; Shuid, A.N.; Das, S.; Soelaiman, I.N. The use of traditional treatment modalities with special mention of Piper sarmentosum in treatment of bone fracture. J. Med. Plants Res. 2011, 5, 71327139. [Google Scholar] [CrossRef]
- Estai, M.A.; Suhaimi, F.H.; Das, S.; Fadzilah, F.M.; Alhabshi, S.M.I.; Shuid, A.N.; Soelaiman, I.N. Piper sarmentosum enhances fracture healing in ovariectomized osteoporotic rats: A radiological study. Clinics 2011, 66, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ramli, E.S.M.; Soelaiman, I.N.; Othman, F.; Ahmad, F.; Shuib, A.N.; Mohamed, N.; Muhammad, N.; Suhaimi, F.H. The effects of Piper sarmentosum water extract on the expression and activity of 11β hydroxysteroid dehydrogenase type 1 in the bones with excessive glucocorticoids. Iran. J. Med. Sci. 2012, 37, 39–46. [Google Scholar]
- Chung, S.; Son, G.H.; Kim, K. Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochim. Biophys. Acta 2011, 1812, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ima, N.S.; Fakhrurazi, H. Palm vitamin E protects bone against Dexamethasone-induced osteoporosis in male rats. Med. J. Malays. 2002, 57, 136–144. [Google Scholar]
- Thaves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-adrenal glucocorticoids and mineralocorticoids: Evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E11–E24. [Google Scholar] [CrossRef] [PubMed]
- Nirwana, S.I.; Suhana, M.R.E.; Fadziyah, M.A.S.; Farihah, H.S.; Fairus, A.; Alfakri, M.N.M. Piper sarmentosum improves bone structure and biomechanical strength of rats given excess glucocorticoid. Br. J. Pharm. Res. 2012, 2, 168–187. [Google Scholar] [CrossRef]
- Fadziyah, M.A.S.; Suhana, M.R.E.; Nirwana, S.I.; Alfakry, M.N.M.; Hamid, A.R.A.; Farihah, H.S. Relationship of osteoblast and osteoclast-related mRNA expression with the use of Piper sarmentosum water extract in the treatment of glucocorticoid-induced osteoporosis. Int. J. Basic Appl. Sci. 2016, 4, 18–32. [Google Scholar]
- Suhana, M.R.E.; Farihah, H.S.; Faizah, O.; Nazrun, S.A.; Norazlina, M.; Norliza, M.; Nirwana, S.I. Piper sarmentosum prevents glucocorticoid-induced osteoporotic bone resorption by increasing 11β hydroxysteroid dehydrogenase type 1 activity. Clin. Ter. 2011, 162, 313–318. [Google Scholar] [PubMed]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11-Hydroxysteroid dehydrogenase type 1: A tissue specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L. The effect of glucocorticoids on bone and muscle. Osteoporos. Sarcopenia 2015, 1, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandic, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [CrossRef] [PubMed]
- Sumazian, Y.; Syahida, A.; Hakiman, M.; Maziah, M. Antioxidant activities, flavonoids, ascorbic acid and phenolic contents of Malaysian vegetables. J. Med. Plant Res. 2010, 4, 881–890. [Google Scholar]
- Estai, M.A.; Soelaiman, I.N.; Shuid, A.N.; Das, S.; Ali, A.M.; Suhaimi, F.H. Histological changes in the fracture callus following the administration of water extract of Piper sarmentosum (daun kadok) in estrogen-deficient rats. Iran. J. Med. Sci. 2011, 36, 281–288. [Google Scholar] [PubMed]
- Mohd Zainudin, M.; Zakaria, Z.; Nordin, N.A.M.M.; Othman, F. Does oral ingestion of Piper sarmentosum cause toxicity in experimental animals? Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M. Methods in Bone Biology in Animals: Biochemical Markers. In Osteoporosis Research; Duque, G., Watanabe, K., Eds.; Springer: London, UK, 2011; pp. 57–82. [Google Scholar]
- Lucinda, L.M.F.; Vieira, B.J.; Oliveira, T.T.; Sá, R.C.S.; Peters, V.M.; Reis, J.E.P.; Guerra, M.O. Evidences of osteoporosis improvement in Wistar rats treated with Ginkgo biloba extract: A histomorphometric study of mandible and femur. Fitoterapia 2010, 81, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Zardooz, H.; Rostamkhani, F.; Zaringhalam, J.; Faraji Shahrivar, F. Plasma corticosterone, insulin and glucose changes induced by brief exposure to Isoflurane, Diethyl ether and CO2 in male rats. Physiol. Res. 2010, 59, 973–978. [Google Scholar] [PubMed]
- Tagawa, N.; Ikariko, N.; Fukumura, K.; Kobayashi, Y. Development of an enzyme-linked immunosorbent assay for serum 11-dehydrocorticosterone in rat and mouse. Biol. Pharm. Bull. 2007, 30, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Asri, S.F.; Mohd Ramli, E.S.; Soelaiman, I.N.; Mat Noh, M.A.; Abdul Rashid, A.H.; Suhaimi, F. Piper sarmentosum Effects on 11β-Hydroxysteroid Dehydrogenase Type 1 Enzyme in Serum and Bone in Rat Model of Glucocorticoid-Induced Osteoporosis. Molecules 2016, 21, 1523. https://doi.org/10.3390/molecules21111523
Mohamad Asri SF, Mohd Ramli ES, Soelaiman IN, Mat Noh MA, Abdul Rashid AH, Suhaimi F. Piper sarmentosum Effects on 11β-Hydroxysteroid Dehydrogenase Type 1 Enzyme in Serum and Bone in Rat Model of Glucocorticoid-Induced Osteoporosis. Molecules. 2016; 21(11):1523. https://doi.org/10.3390/molecules21111523
Chicago/Turabian StyleMohamad Asri, Siti Fadziyah, Elvy Suhana Mohd Ramli, Ima Nirwana Soelaiman, Muhamad Alfakry Mat Noh, Abdul Hamid Abdul Rashid, and Farihah Suhaimi. 2016. "Piper sarmentosum Effects on 11β-Hydroxysteroid Dehydrogenase Type 1 Enzyme in Serum and Bone in Rat Model of Glucocorticoid-Induced Osteoporosis" Molecules 21, no. 11: 1523. https://doi.org/10.3390/molecules21111523





