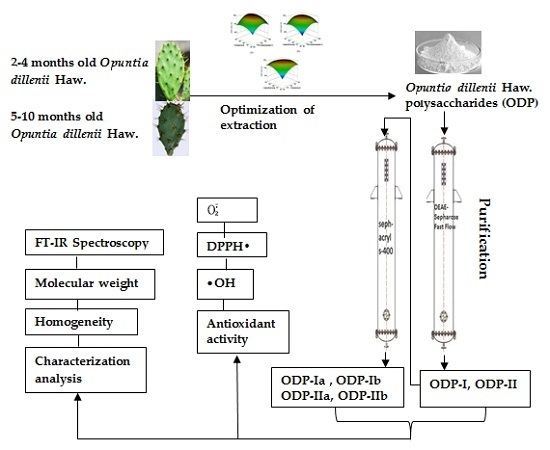
Extraction of Opuntia dillenii Haw. Polysaccharides and Their Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
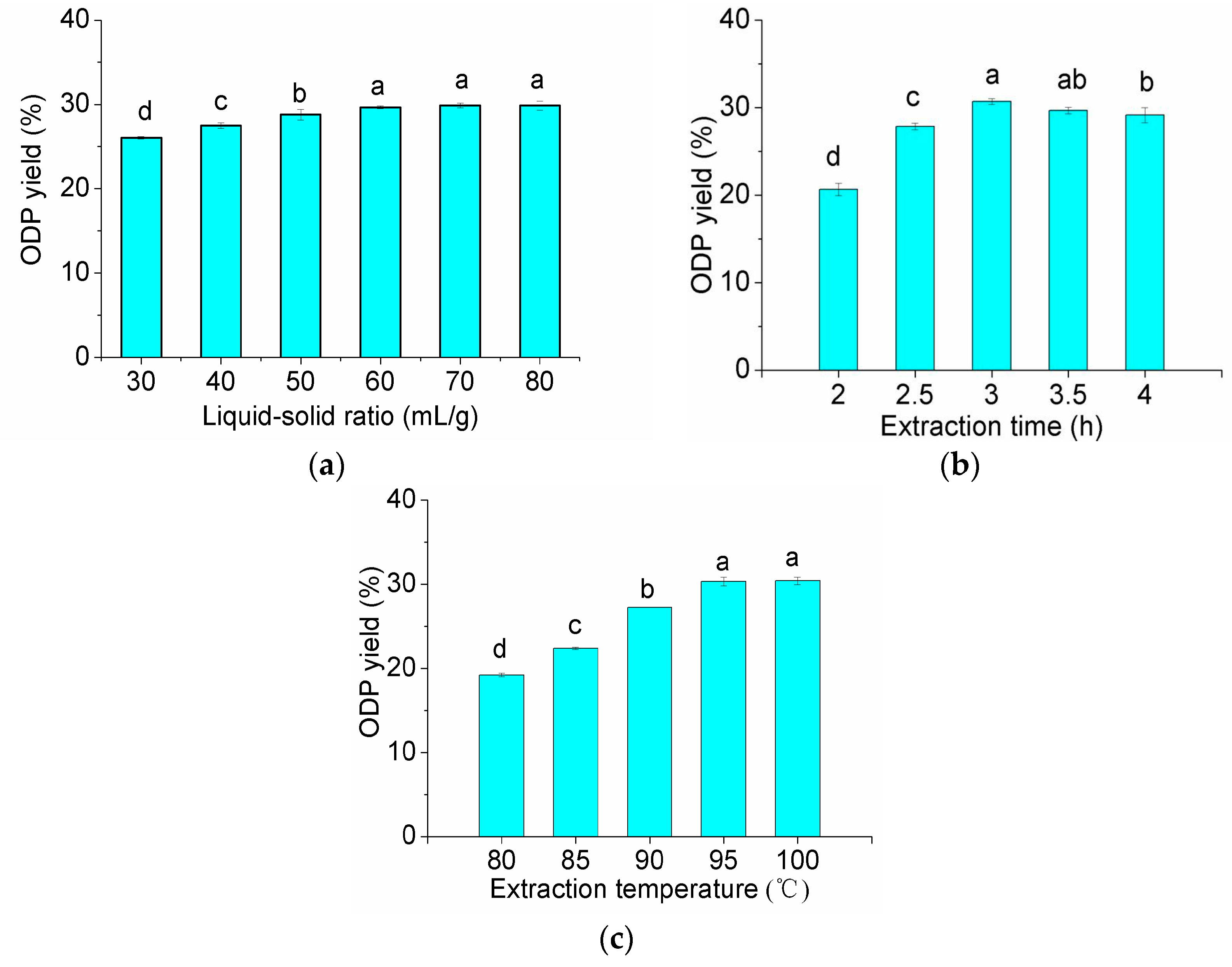
2.1. Optimization of Extraction Conditions for ODP
2.2. Comparison of Extraction Results of T-ODP and O-ODP
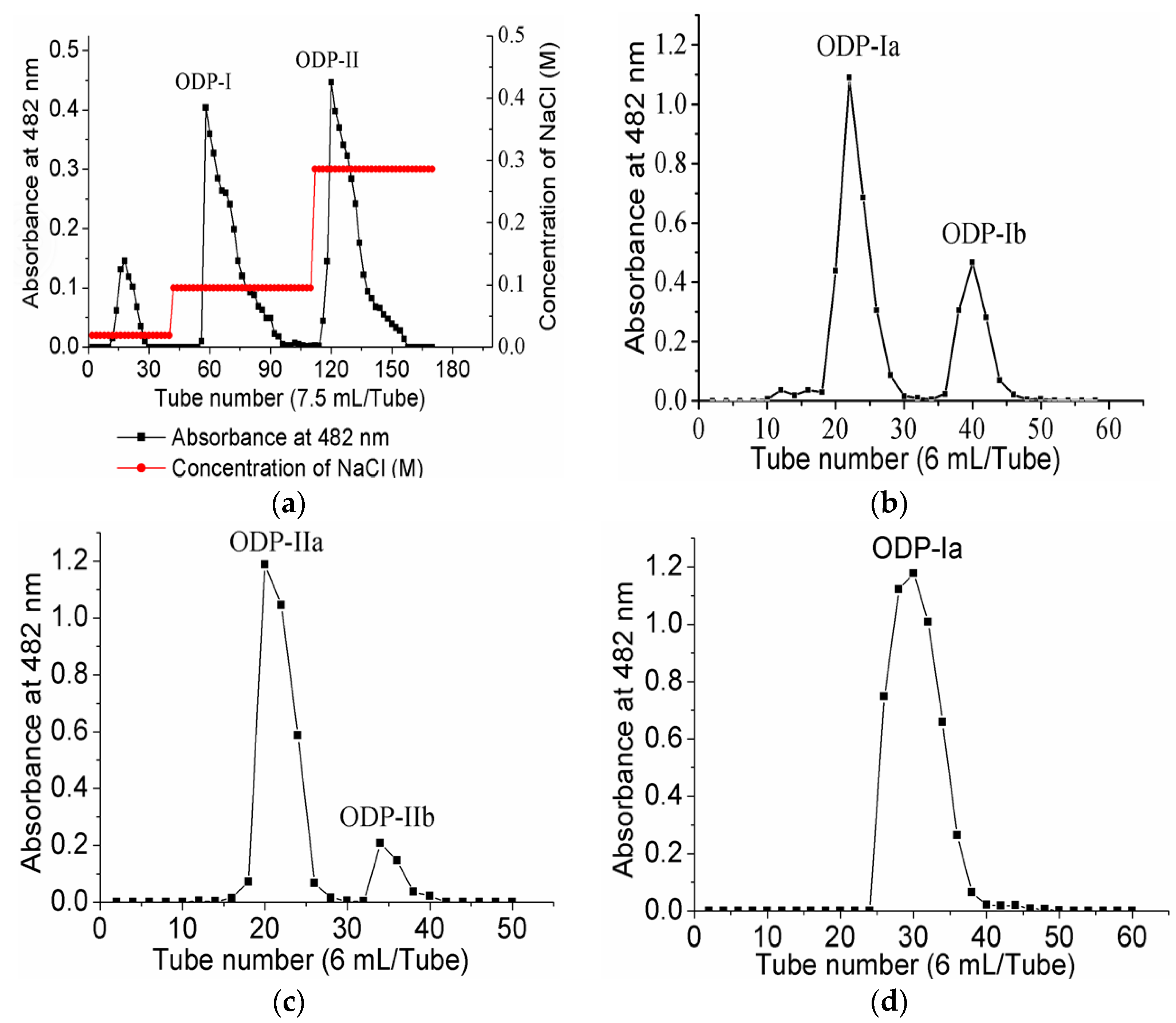
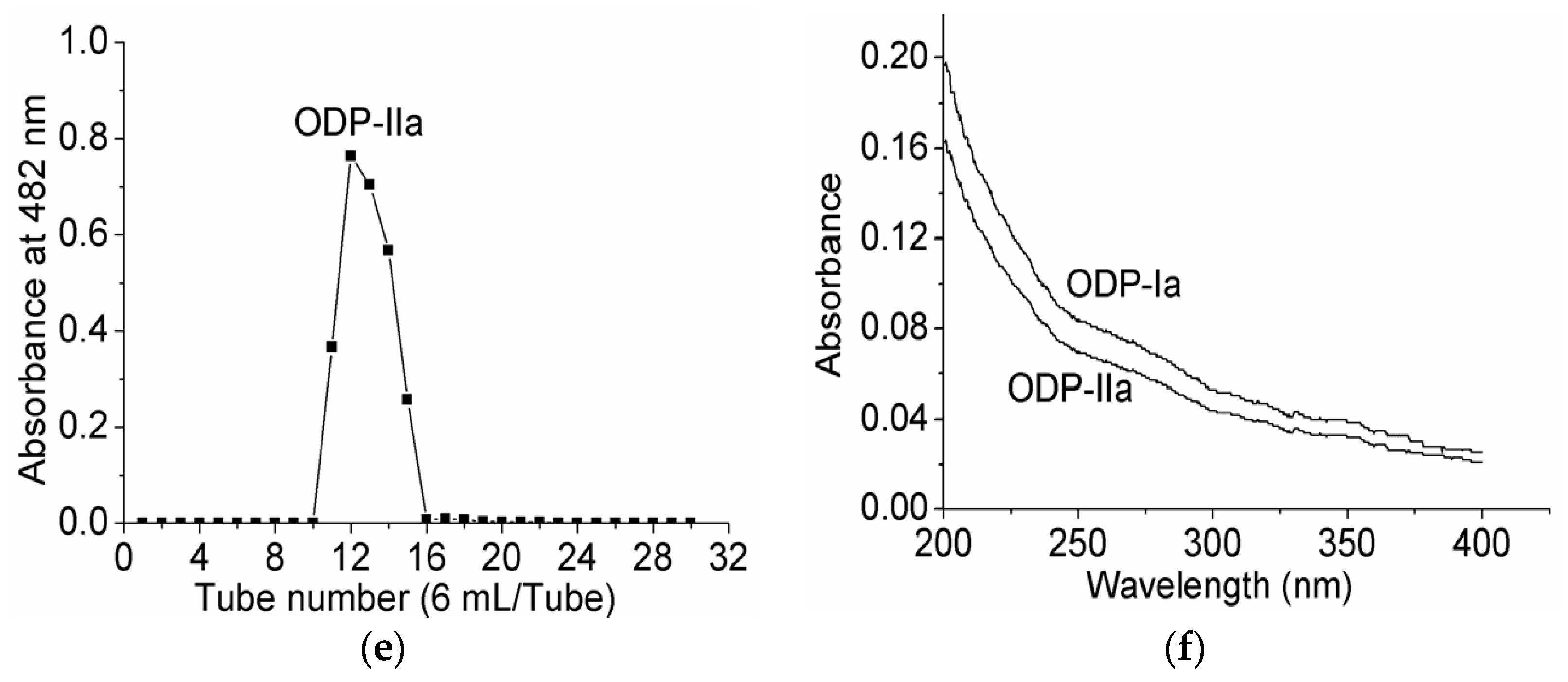
2.3. Homogeneity and Molecular Weight Analysis
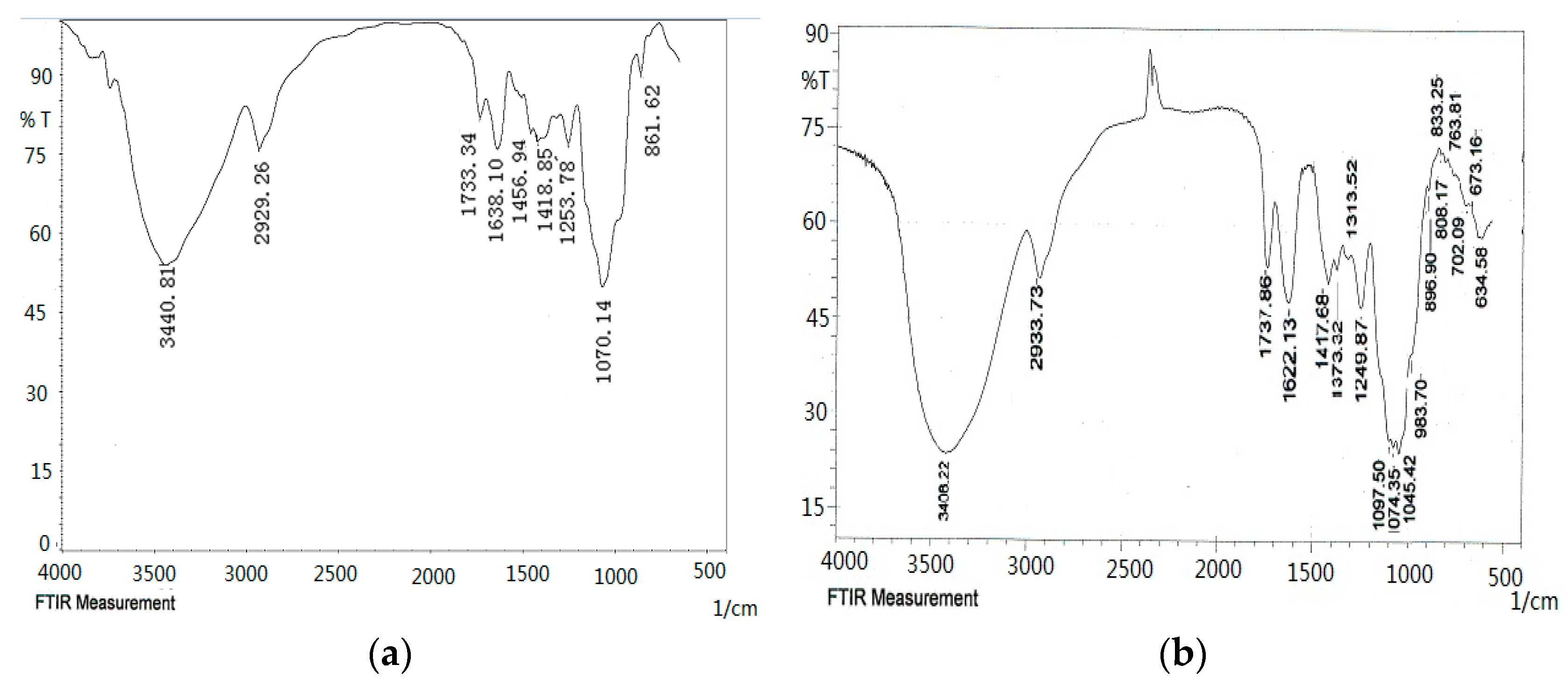
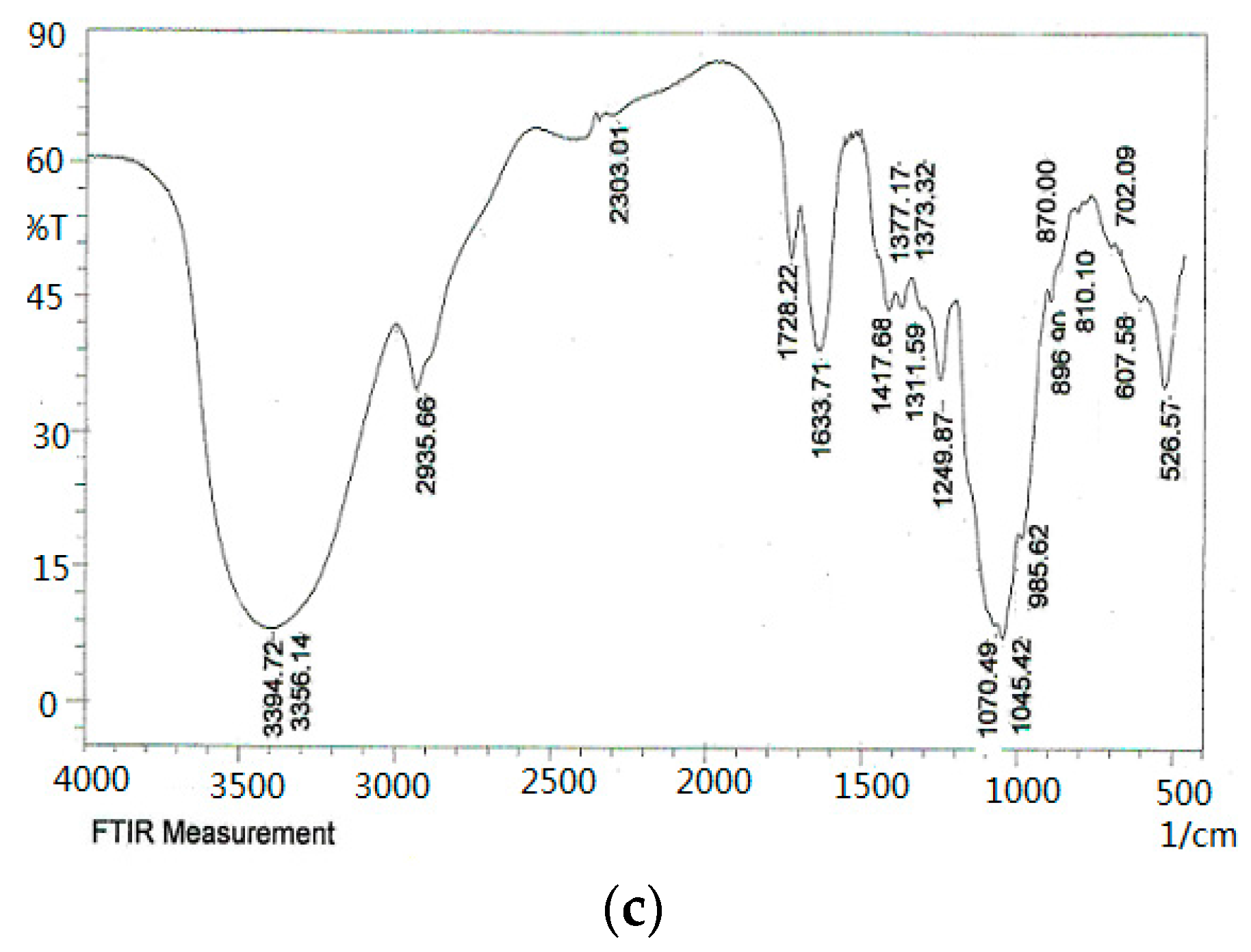
2.4. FT-IR Spectroscopy
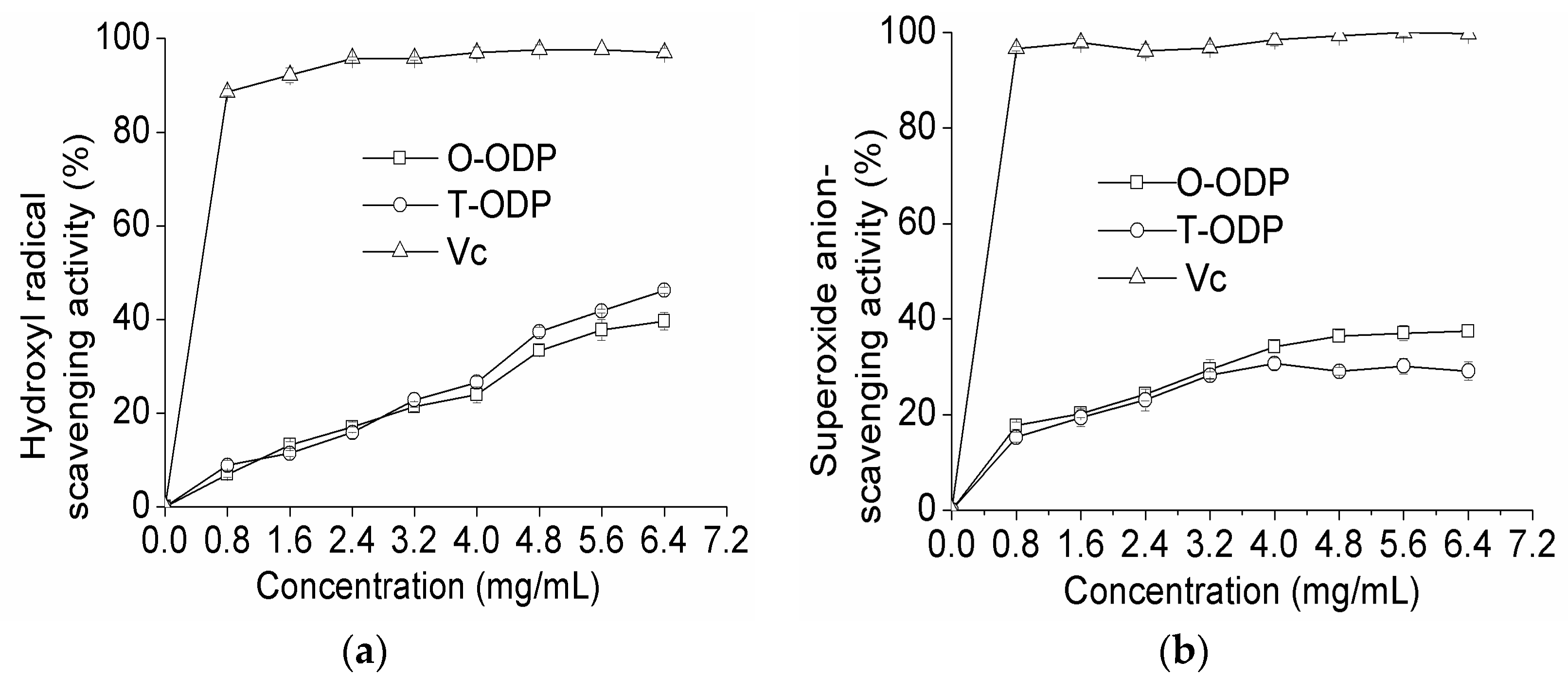
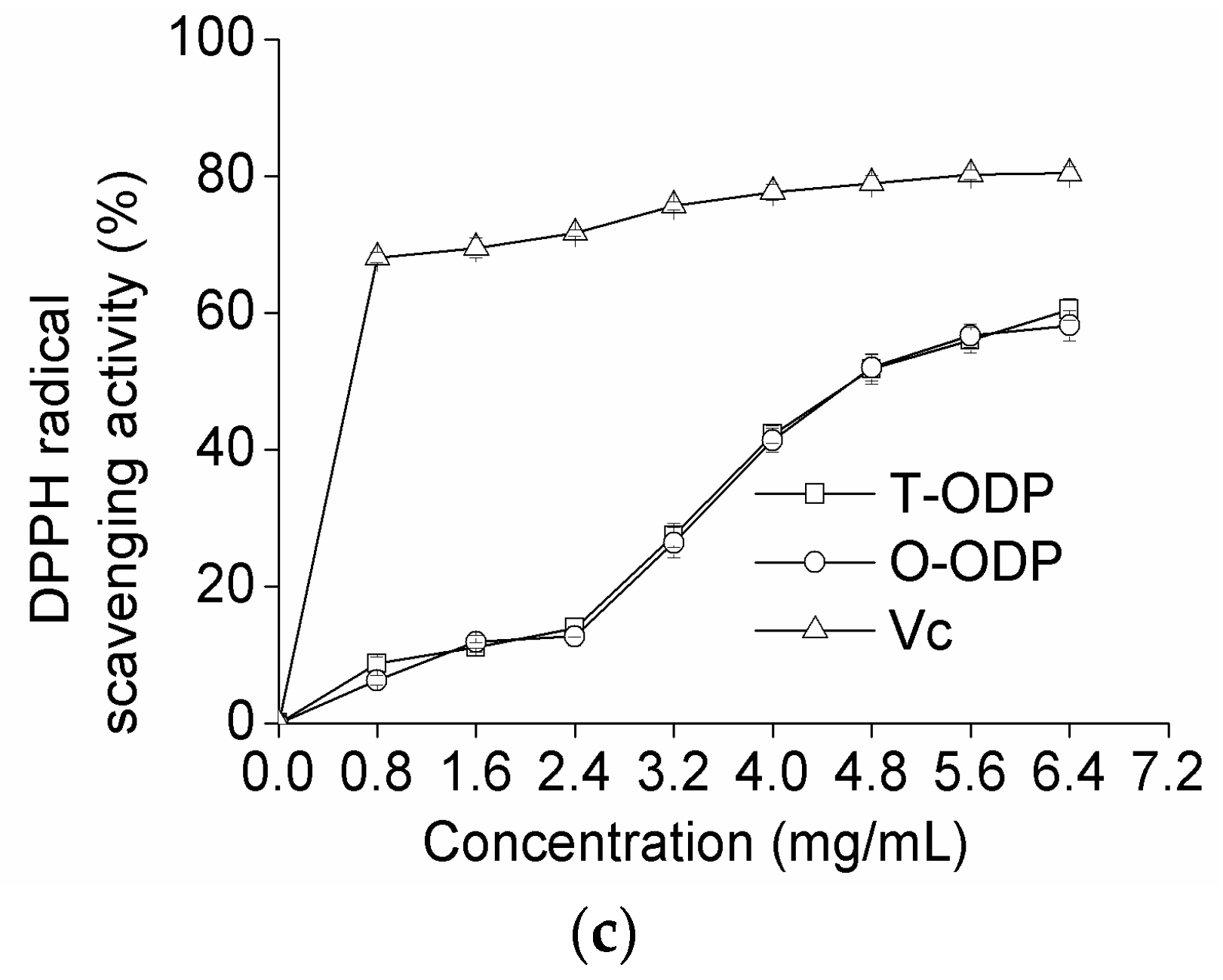
2.5. Antioxidant Activity of ODP
3. Materials and Methods
3.1. Chemicals and Material
3.2. Extraction of ODP
3.3. Optimization of the Extraction Conditions for ODP
3.4. ODP Extraction from Tender and Old Opuntia dillenii Haw.
3.5. Purification of the Polysaccharide
3.6. Characterization of ODP and Its Purified Fractions
3.6.1. Molecular Weight
3.6.2. UV-Vis spectrophotometric Analysis
3.6.3. FT-IR Spectroscopy Analysis
3.7. In Vitro Antioxidant Activity
3.7.1. Scavenging Activity on DPPH Radical
3.7.2. Scavenging Activity on Hydroxyl Radical
3.7.3. Scavenging Activity on Superoxide Radical
3.8. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, Y.; Wang, Y.B.; Jiang, Y.; Prasad, K.N.; Yang, J.; Qu, H. Structure identification of a polysaccharide purified from lyceum barbarium fruit. Int. J. Biol. Macromol. 2015, 82, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Q.; Gizaw, Y.; BeMiller, J.N. Extraction of polysaccharides from a species of Chlorella. Carbohydr. Polym. 2012, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ursekar, B.M.; Soni, P.S.; Date, A.A.; Nagarsenker, M.S. Characterization of soy polysaccharide and its in vitro and in vivo evaluation for application in colon drug delivery. AAPS PharmSciTech 2012, 13, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, Y.; Ren, G. Immunoregulatory activities of polysaccharides from mung bean. Carbohydr. Polym. 2016, 139, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Huang, W.; Yuan, Q.X.; Cheng, J.; Huang, Z.C.; Ouyang, L.J.; Zeng, F.H. Hypolipidemic effects and mechanisms of the main component of Opuntia dillenii Haw. polysaccharides in high-fat emulsion-induced hyperlipidemic rats. Food Chem. 2012, 134, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Hsieh, C.L.; Yen, G.C. The protective effect of Opuntia dillenii Haw fruit against low-density lipoprotein peroxidation and its active compounds. Food Chem. 2008, 106, 569–575. [Google Scholar] [CrossRef]
- Medina, E.M.D.; Rodríguez, E.M.R.; Romero, C.D. Chemical characterization of Opuntia dillenii, and opuntia ficus indica fruits. Food Chem. 2007, 103, 38–45. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Lan, Q.J.; Huang, Z.C.; Ouyang, L.J.; Zeng, F.H. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine 2011, 18, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.R.; Gu, X.H.; Tang, J. Extraction, purification, and characterization of the Polysaccharides from Opuntia Milpa Alt. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.M.; Yang, N.; Yang, B. Optimization of the extraction technique of opuntia polysaccharides via response surface method. Food Mach. 2006, 22, 42–44. [Google Scholar]
- Yan, Y.; Yu, C.; Chen, J.; Li, X.; Wang, W.; Li, S. Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica. Carbohydr. Polym. 2011, 83, 217–224. [Google Scholar] [CrossRef]
- Xia, Y.G.; Kuang, H.X.; Yang, B.Y.; Wang, Q.H.; Liang, J.; Sun, Y.P.; Wang, Y.H. Optimum extraction of acidic polysaccharides from the stems of Ephedra sinica Stapf by Box–Behnken statistical design and its anti-complement activity. Carbohydr. Polym. 2011, 84, 282–291. [Google Scholar] [CrossRef]
- Chen, C.; Kasimu, R.; Xie, X.; Zheng, Y.; Ding, W. Optimised extraction of Erythronium sibiricum bulb polysaccharides and evaluation of their bioactivities. Int. J. Biol. Macromol. 2015, 82, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, S.; Moon, B. Optimization by response surface methodology of lutein recovery from paprika leaves using accelerated solvent extraction. Food Chem. 2016, 205, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pujari, V.; Chandr, T.S. Statistical optimization of medium components for enhanced riboflavin production by a UV-mutant of Eremothecium ashbyii. Process Biochem. 2000, 36, 31–37. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.; Liu, T.; Hu, Y.; Zheng, Q.; Wang, B. Separation, characterization and anticancer activities of a sulfated polysaccharide from Undaria pinnatifida. Int. J. Biol. Macromol. 2015, 83, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.N.; Lu, Y.; Zou, Y.F.; Shen, H. Separation, purification and preliminary structure analysis of acidic polysaccharides from safflower. Eur. Food Res. Technol. 2013, 237, 449–455. [Google Scholar] [CrossRef]
- Li, F.; Gao, J.; Xue, F.; Yu, X.; Shao, T. Extraction Optimization, Purification and physicochemical properties of polysaccharides from Gynura medica. Molecules 2016, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.A.; Barros, A.; Barros, M.; Rutledge, D.N.; Delgadillo, I. Multivariate analysis of uronic acid and neutral sugars in whole pectic samples by FT-IR spectroscopy. Carbohydr. Polym. 1998, 37, 241–248. [Google Scholar] [CrossRef]
- Barker, S.A.; Bourne, E.J.; Stacey, M. Infra-red spectra of carbohydrates. Part I. Some derivatives of d-glucopyranose. J. Chem. Soc. 1954, 171–176. [Google Scholar] [CrossRef]
- Rolin, D.; Tacon, F.L.; Larher, F. Extraction and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohydr. Polym. 2013, 94, 731–736. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, C.; Yu, Z.; Ling, Z.; Li, X.; Yu, Y. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016, 194, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.T.; Gao, T.T.; Li, Z.H.; Ji, L.; Yang, W.; Jeff, B.; Liu, E.X.; Zhou, Y.F. Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries). Food Chem. 2013, 138, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Asker, M.M.; Manal, G.M.; Ghada, S.I. Structural characterization and biological activity of acidic polysaccharide fractions isolated from Bacillus polymyxa NRC-A. J. Appl. Sci. Res. 2007, 3, 1170–1177. [Google Scholar]
- Yu, P.; Zhang, Q.; Li, N.; Xu, Z.; Wang, Y.; Li, Z. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Ye, H.; Hu, B.; Li, Z.; Jabbar, S. Optimization of extraction, characterization and antioxidant activity of polysaccharides from Brassica rapa L. Int. J. Biol. Macromol. 2015, 82, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Mao, W.J.; Chen, Y.; Gao, S.D.; Li, H.Y.; Qi, X.H.; Chen, Y.L.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Quan, H.; Yu, Q.Y.; Shi, J.; Xiong, C.Y.; Ling, Z.J.; He, P.M. Structural characterization and antioxidant activities of 2 water-soluble polysaccharide fractions purified from tea (Camellia sinensis) flower. J. Food Sci. 2011, 76, C462–C471. [Google Scholar] [CrossRef] [PubMed]
- You, L.J.; Gao, Q.; Feng, M.Y.; Yang, B.; Ren, J.Y.; Gu, J.Y.; Cui, C.H.; Zhao, M.M. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, G.; Suarez, N.; Bessio, M.I.; Ferreira, F.; Massaldi, H. Quantitative determination of pneumococcal capsular polysaccharide serotype 14 using a modification of phenol-sulfuric acid method. J. Microbiol. Methods 2003, 52, 69–73. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, B.; Wang, J.; Li, B.; Jiang, Y. Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem. 2006, 98, 539–544. [Google Scholar] [CrossRef]
- Zhang, S.S.; Liu, X.Q.; Yan, L.H.; Zhang, Q.W.; Zhu, J.J.; Huang, N.; Wang, Z.M. Chemical compositions and antioxidant activities of polysaccharides from the sporophores and cultured products of Armillaria mellea. Molecules 2015, 20, 5680–5697. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Chen, L.Y. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Food Res. 2013, 57, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Shen, M.Y.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Li, C.; Huang, D.F.; Wang, Y.X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
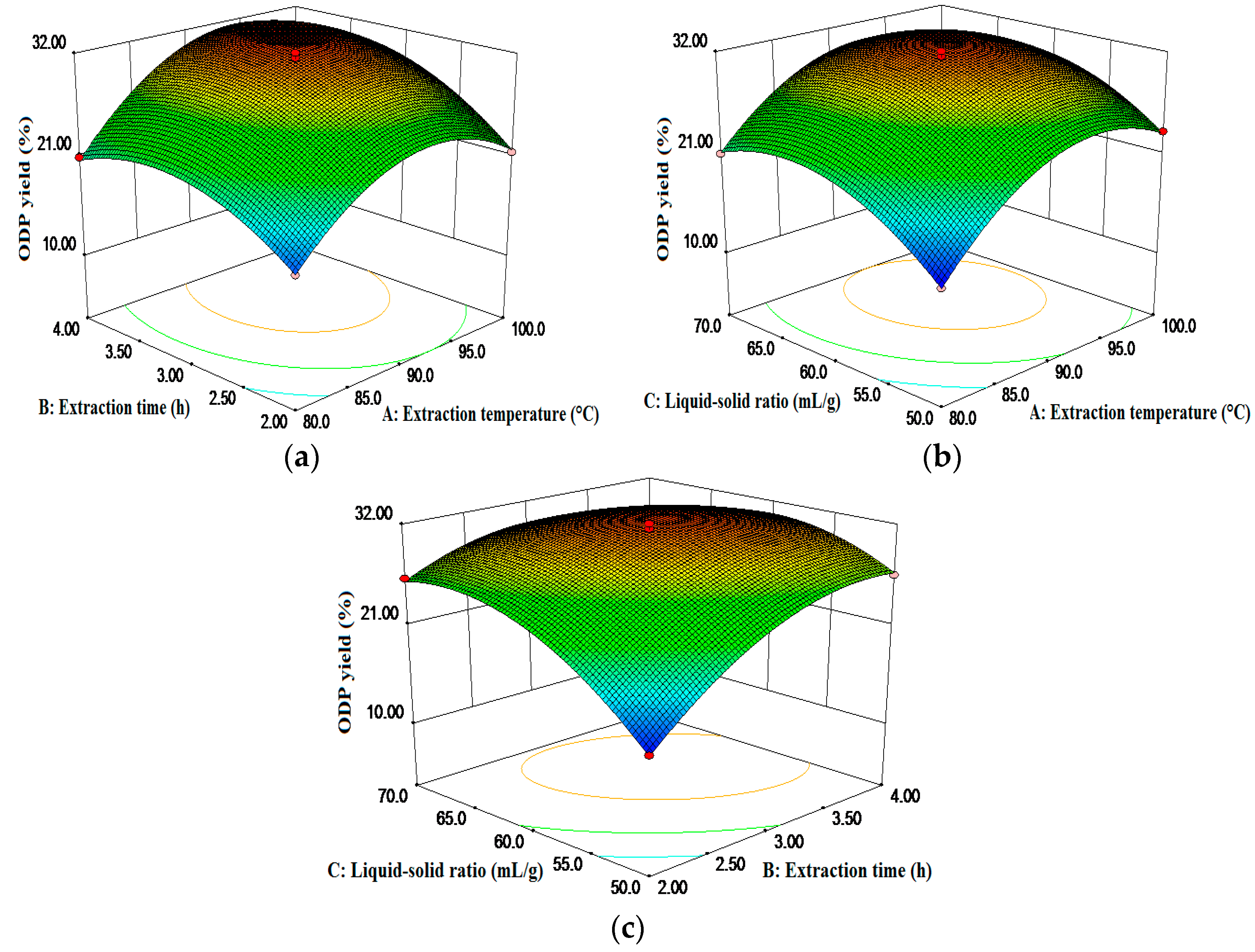
| No. | A | B | C | ODP Yield (%) | |
|---|---|---|---|---|---|
| Extraction Temperature (°C) | Extraction Time (h) | Liquid-Solid Ratio (mL/g) | Experimental | Predicted | |
| 1 | 90 | 3 | 60 | 30.45 | 31.29 |
| 2 | 90 | 3 | 60 | 31.45 | 31.29 |
| 3 | 80 | 2 | 60 | 16.53 | 16.59 |
| 4 | 90 | 2 | 50 | 15.27 | 15.20 |
| 5 | 100 | 4 | 60 | 29.55 | 29.48 |
| 6 | 80 | 3 | 50 | 14.95 | 14.95 |
| 7 | 90 | 3 | 60 | 33.05 | 31.29 |
| 8 | 90 | 4 | 70 | 25.97 | 26.04 |
| 9 | 90 | 4 | 50 | 26.54 | 26.80 |
| 10 | 90 | 3 | 60 | 30.4 | 31.29 |
| 11 | 90 | 3 | 60 | 31.11 | 31.29 |
| 12 | 90 | 2 | 70 | 26.14 | 25.88 |
| 13 | 100 | 3 | 50 | 23.46 | 23.26 |
| 14 | 80 | 2 | 60 | 21.45 | 21.72 |
| 15 | 80 | 3 | 70 | 21.02 | 21.21 |
| 16 | 100 | 3 | 70 | 26.93 | 26.93 |
| 17 | 80 | 4 | 60 | 20.85 | 20.58 |
| Source | SS | Ms | F-Value | Prob > F | Significance |
|---|---|---|---|---|---|
| Model | 542.07 | 60.23 | 83.93 | <0.0001 | ** |
| A | 98.28 | 98.28 | 136.95 | <0.0001 | ** |
| B | 69.15 | 69.15 | 96.36 | <0.0001 | ** |
| C | 49.20 | 49.2 | 68.57 | <0.0001 | ** |
| AB | 3.57 | 3.57 | 4.98 | 0.0609 | |
| AC | 1.69 | 1.69 | 2.36 | 0.1688 | |
| BC | 32.72 | 32.72 | 45.59 | 0.0003 | ** |
| A2 | 129.39 | 129.39 | 180.31 | <0.0001 | ** |
| B2 | 56.2 | 56.2 | 78.32 | <0.0001 | ** |
| C2 | 72.81 | 72.81 | 101.47 | <0.0001 | ** |
| Residual | 5.02 | 0.72 | |||
| Lack of fit | 0.37 | 0.12 | 0.11 | 0.9522 | |
| Pure error | 4.65 | 1.16 | |||
| Cortotal | 547.09 |
| Type | ODP Yield (%) | Purity (%) | Protein Content (%) |
|---|---|---|---|
| 2–4 month-old Opuntia dillenii Haw. | 30.60 ± 0.40 a | 85.32 ± 0.32 a | 0.65 ± 0.09 a |
| 5–10 month-old Opuntia dillenii Haw. | 18.97 ± 0.58 b | 57.99 ± 0.89 b | 0.87 ± 0.12 a |
| Samples | Antioxidant Activity (%) | ||
|---|---|---|---|
| DPPH Radical | Hydroxyl Radical | Superoxide Radical | |
| T-ODP | 54.45 ± 1.46 c | 40.78 ± 0.56 c,d | 36.88 ± 0.99 c |
| ODP-I | 46.17 ± 1.67 d | 39.83 ± 0.90 c,d | 36.50 ± 0.89 c |
| ODP-Ia | 58.44 ± 1.37 b | 45.69 ± 0.92 b | 43.71 ± 1.30 b |
| ODP-Ib | 15.29 ± 0.56 f | 12.47 ± 0.30 e | 13.37 ± 0.41 e |
| ODP-II | 33.92 ± 1.14 e | 39.07 ± 1.46 d | 30.01 ± 0.21 d |
| ODP-IIa | 45.55 ± 0.89 d | 41.47 ± 1.18 c | 37.19 ± 0.57 c |
| ODP-IIb | 8.80 ± 0.74 g | 0.84 ± 0.12 f | 5.22 ± 0.12 f |
| Vc | 80.06 ± 0.85 a | 96.35 ± 0.98 a | 99.13 ± 0.09 a |
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: Extraction temperature (°C) | 80 | 90 | 100 |
| B: Liquid-solid ratio (mL/g) | 50 | 60 | 70 |
| C: Extraction time (h) | 2 | 3 | 4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yuan, Q.; Zhou, X.; Zeng, F.; Lu, X. Extraction of Opuntia dillenii Haw. Polysaccharides and Their Antioxidant Activities. Molecules 2016, 21, 1612. https://doi.org/10.3390/molecules21121612
Li H, Yuan Q, Zhou X, Zeng F, Lu X. Extraction of Opuntia dillenii Haw. Polysaccharides and Their Antioxidant Activities. Molecules. 2016; 21(12):1612. https://doi.org/10.3390/molecules21121612
Chicago/Turabian StyleLi, Heng, Qingxia Yuan, Xianjiao Zhou, Fuhua Zeng, and Xiangyang Lu. 2016. "Extraction of Opuntia dillenii Haw. Polysaccharides and Their Antioxidant Activities" Molecules 21, no. 12: 1612. https://doi.org/10.3390/molecules21121612