Design, Synthesis and Biological Evaluation of Novel Primaquine-Cinnamic Acid Conjugates of the Amide and Acylsemicarbazide Type
Abstract
:1. Introduction
2. Results and Discussion
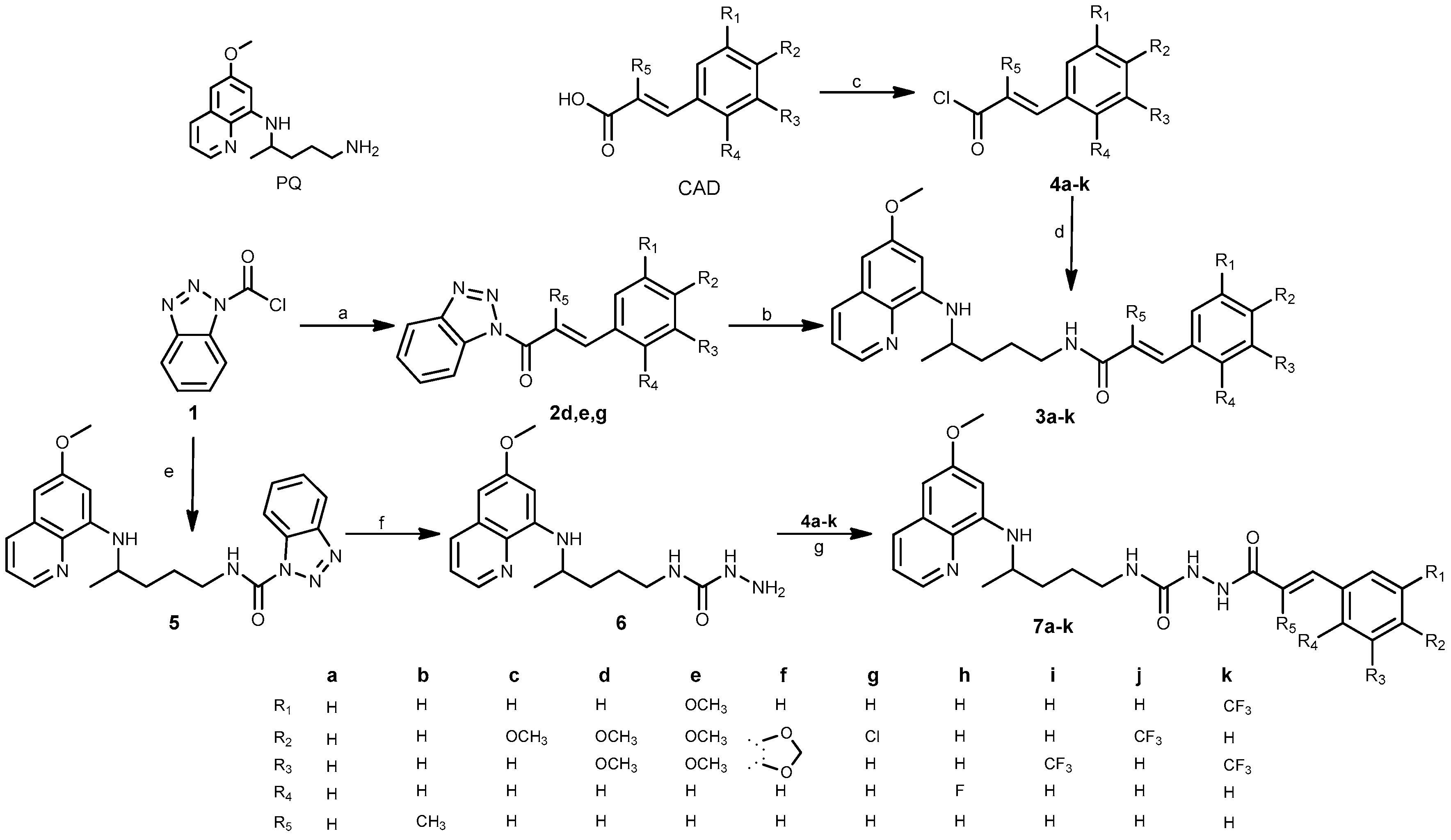
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Anticancer Activity
2.2.2. Antiviral Activity Assays
2.2.3. Cellular Cytotoxicity Assays
2.2.4. Antioxidative Activity
3. Experimental Section
3.1. Chemistry
3.1.1. Materials and Methods
3.1.2. General Procedure for the Synthesis of Benzotriazolides 2d,e,g
3.1.3. General Procedure for the Synthesis of Chlorides 4a–k
3.1.4. General Procedure for the Synthesis of Amides 3a–k
3.1.5. Synthesis of N-(4-((6-Methoxyquinolin-8-yl)amino)pentyl)-1H-benzo[d][1,2,3]triazole-1-carboxamide (5)
3.1.6. Synthesis of N-(4-((6-Methoxyquinolin-8-yl)amino)pentyl)hydrazinecarboxamide (6)
3.1.7. General Procedure for the Synthesis of Acylsemicarbazides 7a–k
3.2. Biological Evaluation
3.2.1. Anticancer Activity
3.2.2. Antiviral Activity
3.2.3. Cytotoxicity Assays
3.2.4. Interaction of the New Derivatives with the Stable Radical DPPH
3.2.5. Inhibition of Linoleic Acid Peroxidation
3.2.6. Anti-inflammatory Activity: LOX Inhibition Study In Vitro
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAPH | 2,2’-azobis(2-amidinopropane) dihydrochloride |
| BtcCl | 1-benzotriazole carboxylic acid chloride |
| BtH | 1H-benzo[d][1,2,3]triazole |
| CA | cinnamic acid |
| CAD | cinnamic acid derivative |
| CEM | acute lymphoblastic leukemia cell line |
| CIS | cisplatin |
| DMEM | Dulbecco′s modified Eagle′s medium |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC50 | concentration required to reduce virus-induced cytopathogenicity by 50% |
| FBS | fetal bovine serum |
| 5-FU | 5-fluorouracil |
| H460 | lung carcinoma cell line |
| HEL | human erythroleukemia cell line |
| HeLa | cervical carcinoma cell line |
| IC50 | concentration that causes 50% growth inhibition |
| L1210 | murine lymphocytic leukemia cell line |
| LOX | soybean lipoxygenase |
| LP | lipid peroxidation |
| MCC | minimum cytotoxic concentration, concentration that causes a microscopically detectable alteration of normal cell morphology |
| MCF-7 | breast adenocarcinoma cell line |
| MR | molar refractivity |
| MTT | (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NDGA | nordihydroguaiaretic acid |
| PSA | molecular polar surface area |
| PG | percentage of growth |
| PQ | primaquine |
| RA | DPPH reducing ability |
| SI | selectivity ratio, MCC to EC50 ratio |
| SOR | sorafenib |
| SW 620 | colon cancer cell line |
| TEA | triethylamine |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| UDA | Urtica dioica agglutinin |
References
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 292–349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.J. Cinnamic acid derivatives: A new chapter of various pharmacological activities. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Lone, R.; Shuab, R.; Koul, K.K. Role of cinnamate and cinnamate derivatives in pharmacology. Glob. J. Pharmacol. 2014, 8, 328–335. [Google Scholar]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, amides and substituted derivatives of cinnamic acid: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2004, 39, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Campbell, B.C.; Mahomey, N.E.; Chan, K.L.; Molyneux, R.J. Identification of phenolics for control of Aspergillus flavus using Saccharomyces cerevisiae in a model target-gene bioassay. J. Agric. Food Chem. 2004, 52, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, H.; Kobamoto, N.; Yasuda, M.; Tawata, S. Fungitoxic and phytotoxic activities of cinnamic acid esters and amides. J. Pestic. Sci. 2000, 25, 263–266. [Google Scholar] [CrossRef]
- Neogi, P.; Lakner, F.J.; Medicherla, S.; Cheng, J.; Dey, D.; Gowri, M.; Nag, B.; Sharma, S.D.; Pickford, L.B.; Gross, C. Synthesis and structure-activity relationship studies of cinnamic acid-based novel thiazolidinedione antihyperglycemic agents. Bioorg. Med. Chem. 2003, 11, 4059–4067. [Google Scholar] [CrossRef]
- Bairwa, R.; Kakwani, M.; Tawari, N.R.; Lalchandani, J.; Ray, M.K.; Rajan, M.G.R.; Degani, M.S. Novel molecular hybrids of cinnamic acids and guanylhydrazones as potential antitubecular agents. Bioorg. Med. Chem. 2010, 20, 1623–1625. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.M.; Nadadhur, G.; Daneluzzi, D.; Dimova, V.; Gangadharam, P.R. Antimycobacterial activity of a new rifamycin derivative, 3-(4-cinnamylpiperazinyl iminomethyl) rifamycin SV (T9). J. Antimicrob. Agents Chemother. 1995, 39, 2320–2324. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.; Lourenco, M.C.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Kakwani, M.D.; Suryavanshi, P.; Ray, M.; Rajan, M.G.R.; Majee, S.; Samad, A.; Devarajan, P.; Degani, M.S. Design, synthesis and antimycobacterial activity of cinnamide derivatives: A molecular hybridization approach. Bioorg. Med. Chem. Lett. 2011, 21, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Yoya, G.K.; Bedos-Belval, F.; Constant, P.; Duran, H.; Daffé, M.; Baltas, M. Synthesis and evaluation of a novel series of pseudo-cinnamic derivatives as antituberculosis agents. Bioorg. Med. Chem. Lett. 2009, 19, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Bogdashev, N.N.; Tukhovskaya, N.A.; Pogrebnyak, A.V. Physicochemical characterisation of cinnamic acid derivatives. Part 1. Relationship between antioxidant activity and physicochemical properties. Pharm. Chem. J. 1998, 32, 31–33. [Google Scholar]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Arya, P.; Mukherjee, C.; Singh, B.K.; Singh, N.; Parmar, V.S.; Prasad, A.K.; Ghosh, B. Novel aromatic ester from Piper longum and its analogues inhibit expression of cell adhesion molecules on endothelial cells. Biochemistry 2005, 44, 15944–15952. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Choudhary, R.K. Process for Preparation of Sunscreen Agents. U.S. Patent 5,527,947, 19 December 1996. [Google Scholar]
- Fernandez-Martinez, E.; Bobadilla, R.A.; Morales-Rios, M.S.; Muriel, P.; Perez-Alvarez, V.M. Trans-3-phenyl-2-propenoic acid (cinnamic acid) derivatives: Structure-activity relationship as hepatoprotective agents. Med. Chem. 2007, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, Y.B.; Moon, S.S.; Bok, S.H.; Kim, D.J.; Ha, T.Y.; Jeong, T.S.; Jeong, K.S.; Choi, M.S. Hypocholesterolemic and antioxidant properties of 3-(4-hydroxyl)propanoic acid derivatives in high-cholesterol fed rats. Chem. Biol. Interact. 2007, 170, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Yabe, T.; Hirahara, H.; Harada, N.; Ito, N.; Nagal, T.; Sangi, T.; Yamada, H. Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience 2010, 165, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Ginsburg, H. Effects of cinnamic acid derivatives on in vitro growth of Plasmodium falciparum and on the permeability of the membrane of malaria-infected erythrocytes. Antimicrob. Agents Chemother. 1992, 36, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Fernandes, I.; Mateus, N.; Teixeira, C.; Gomes, P. Recycling antimalarial leads for cancer: Antiproliferative properties of N-cinnamoyl chloroquine analogues. Bioorg. Med. Chem. Lett. 2013, 23, 6769–6772. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Teixeira, C.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudencio, M.; Gomes, P. PRIMACINS, N-cinnamoyl-primaquine conjugates, with improved liver-stage antimalarial activity. Med. Chem. Commun. 2012, 3, 1170–1172. [Google Scholar] [CrossRef]
- Pérez, B.; Teixeira, C.; Gomes, A.S.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudęncio, M.; Gomes, P. In vitro efficiency of 9-(N-cinnamoylbutyl)aminoacridines against blood- and liver-stage malaria parasites. Bioorg. Med. Chem. Lett. 2013, 23, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Teixeira, C.; Figueiras, M.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Novel cinnamic acid/4-aminoquinoline conjugates bearing non-proteinogenic amino acids: Towards the development of potential dual action antimalarials. Eur. J. Med. Chem. 2012, 54, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Chang, X.; Zhang, C.; Zhou, H.; Liu, M. Ozagrel for acute ischemic stroke: A meta-analysis of data from randomized controlled trials. Neurol. Res. 2012, 34, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Chloroquine and its analogs: A new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 2009, 625, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Hu, C.; Lee, H. Design and synthesis of chloroquine analogs with anti-breast cancer property. Eur. J. Med. Chem. 2010, 45, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Van Huijsduijnen, R.H.; Kiplin Guy, R.; Chibale, K.; Haynes, R.K.; Peitz, I.; Kelter, G.; Phillips, M.A.; Vennerstrom, J.L.; Yuthavong, Y.; Wells, T.N.C. Anticancer properties of distinct antimalarial drug classes. PLoS ONE 2013, 8, e82962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Isaka, Y. Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 2013, 73, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Choudhury, D.; Datta, S.; Bhattacharya, S.; Chakrabarti, G. Inhibition of autophagy by chloroquine potentiates synergistically anti-cancer property of artemisinin by promoting ROS dependent apoptosis. Biochimie 2014, 107, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shang, Y.; Chen, S-Z. Chloroquine potentiates the anti-cancer effect of lidamycin on non-small cell lung cancer cells in vitro. Acta Pharmacol. Sin. 2014, 35, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Aziz, A.; Shouman, S.; El-Demerdash, E.; Elgendy, M.; Abdel-Naim, A.B. Chloroquine as a promising adjuvant chemotherapy together with sunitinib. Sci. Proc. 2014, 1, e384. [Google Scholar]
- Das, A.K. Anticancer effect of antimalarial artemisinin compounds. Ann. Med. Health Sci. Res. 2015, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Coulter, D.W.; Vennerstrom, J.; Sharp, J.G.; Dong, Y.; Wang, X.; McIntyre, E.; McGuire, T. Screening of investigational antimalarials for anticancer activity in high risk N-MYC amplified neuroblastoma (NB). Cancer Res. 2015, 75. [Google Scholar] [CrossRef]
- Xu, C.-C.; Deng, T.; Fan, M.-L.; Lv, W.-B.; Liu, J.-H.; Yu, B.-Y. Synthesis and in vitro antitumor evaluation of dihydroartemisinin-cinnamic acid ester derivatives. Eur. J. Med. Chem. 2016, 107, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Duffy, R.; Wade, C. Discovery of anticancer drugs from antimalarial natural products: A MEDLINE literature review. Drug Discov. Today 2012, 17, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Džimbeg, G.; Zorc, B.; Kralj, M.; Ester, K.; Pavelić, K.; Balzarini, J.; de Clercq, E.; Mintas, M. The novel primaquine derivatives of N-alkyl, cycloalkyl or aryl urea: Synthesis, cytostatic and antiviral activity evaluations. Eur. J. Med. Chem. 2008, 43, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, M.; Perković, I.; Zorc, B.; Ester, K.; Kralj, M.; Hadjipavlou-Litina, D.; Pontiki, E. Urea and carbamate derivatives of primaquine: Synthesis, cytostatic and antioxidant activities. Bioorg. Med. Chem. 2009, 17, 5605–5613. [Google Scholar]
- Perković, I.; Tršinar, S.; Žanetić, J.; Kralj, M.; Martin-Kleiner, I.; Balzarini, J.; Hadjipavlou-Litina, D.; Katsori, A.M.; Zorc, B. Novel 1-acyl-4-substituted semicarbazide derivatives of primaquine—Synthesis, cytostatic, antiviral and antioxidative studies. J. Enzym. Inhib. Med. Chem. 2013, 28, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Pavić, K.; Perković, I.; Cindrić, M.; Pranjić, M.; Martin-Kleiner, I.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Katsori, A.M.; Zorc, B. Novel semicarbazides and ureas of primaquine with bulky aryl or hydroxyalkyl substituents: Synthesis, cytostatic and antioxidative activity. Eur. J. Med. Chem. 2014, 86, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Perković, I.; Antunović, M.; Marijanović, I.; Pavić, K.; Ester, K.; Kralj, M.; Vlainić, J.; Kosalec, I.; Schols, D.; Hadjipavlou-Litina, D.; et al. Novel urea and bis-urea primaquine derivatives with hydroxyphenyl and halogenphenyl substituents: Synthesis and biological evaluation. Eur. J. Med. Chem. 2016, 124, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, R.; Komatsu, K.; Bonaz-Krause, P.; Zyrianov, Y.; McKenna, C.E.; Csipke, C.; Tokes, Z.A.; Lien, E.J. Synthesis, biological evaluation, and quantitative structure-activity relationship analysis of new Schiff Bases of hydroxysemicarbazide as potential antitumor agents. J. Med. Chem. 2002, 45, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Zovko, M.; Zorc, B.; Jadrijević-Mladar Takač, M.; Metelko, B.; Novak, P. The novel ketoprofenamides—Synthesis and spectroscopic characterization. Croat. Chem. Acta 2003, 76, 335–341. [Google Scholar]
- Barbarić, M.; Kralj, M.; Marjanović, M.; Husnjak, I.; Pavelić, K.; Filipović-Grčić, J.; Zorc, D.; Zorc, B. Synthesis and in vitro antitumor effect of diclofenac and fenoprofen thiolated and nonthiolated polyaspartamide-drug conjugates. Eur. J. Med. Chem. 2007, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Rajić, Z.; Butula, I.; Zorc, B.; Kraljević Pavelić, S.; Hock, K.; Pavelić, K.; Naesens, L.; de Clercq, E.; Balzarini, J.; Przyborowska, M.; et al. Cytostatic and antiviral evaluations of NSAID hydroxamic acids. Chem. Biol. Drug Des. 2009, 73, 328–338. [Google Scholar] [PubMed]
- Rajić, Z.; Hadjipavlou-Litina, D.; Pontiki, E.; Kralj, M.; Šuman, L.; Zorc, B. The novel ketoprofen amides—Synthesis and biological evaluation as antioxidants, lipoxygenase inhibitors and cytostatic agents. Chem. Biol. Drug Des. 2010, 75, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Luzina, E.L.; Popov, A.V. Synthesis, evaluation of anticancer activity and COMPARE analysis of N-bis(tri fluoromethyl)alkyl-N’-substituted ureas with pharmacophoricmoieties. Eur. J. Med. Chem. 2012, 53, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Instant Cheminformatics Solutions. Available online: http://www.chemicalize.org/ (accessed on 1 September 2016).
- Rioux, N.; Castonguay, A. Inhibitors of lipoxygenase: A new class of cancer chemopreventive agents. Carcinogenesis (Lond.) 1998, 19, 1393–1400. [Google Scholar] [CrossRef]
- Kort, W.J.; Bijma, A.M.; van Dam, J.J.; van der Ham, A.C.; Hekking, J.M.; van der Ingh, H.F.; Meijer, W.S.; van Wilgenburg, M.G.; Zijlstra, F.J. Eicosanoids in breast cancer patients before and after mastectomy. Prostaglandin Leukot. Essent. 1992, 45, 319–327. [Google Scholar] [CrossRef]
- De Clercq, E.; Holý, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Naesens, L.; Slachmuylders, J.; Niphuis, H.; Rosenberg, I.; Holý, A.; Schellekens, H.; de Clercq, E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS 1991, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
| Compd. | Molecular Formula | Number of Atoms | MW | log P | H-Bond Donor | H-Bond Acceptor | Lipinski Score a | MR (cm3/mol) | PSA (Å2) |
|---|---|---|---|---|---|---|---|---|---|
| 3a | C24H27N3O2 | 56 | 389.49 | 3.82 | 2 | 4 | 4 | 118.37 | 63.25 |
| 3b | C25H29N3O2 | 59 | 403.53 | 4.22 | 2 | 4 | 4 | 122.73 | 63.25 |
| 3c | C25H29N3O3 | 60 | 419.52 | 3.66 | 2 | 5 | 4 | 124.84 | 72.48 |
| 3d | C26H31N3O4 | 64 | 449.54 | 3.51 | 2 | 6 | 4 | 131.30 b | 81.71 |
| 3e | C27H33N3O5 | 68 | 479.57 | 3.35 | 2 | 7 | 4 | 137.76 b | 90.94 |
| 3f | C25H27N3O4 | 59 | 433.50 | 3.44 | 2 | 6 | 4 | 124.14 | 81.71 |
| 3g | C24H26ClN3O2 | 56 | 423.94 | 4.43 | 2 | 4 | 4 | 123.18 | 63.25 |
| 3h | C24H26FN3O2 | 56 | 407.48 | 3.96 | 2 | 4 | 4 | 118.59 | 63.25 |
| 3i | C25H26F3N3O2 | 59 | 457.49 | 4.70 | 2 | 4 | 4 | 124.35 | 63.25 |
| 3j | C25H26F3N3O2 | 59 | 457.49 | 4.70 | 2 | 4 | 4 | 124.35 | 63.25 |
| 3k | C26H25F6N3O2 | 62 | 525.49 | 5.58 | 2 | 4 | 2 | 130.32 | 63.25 |
| 7a | C25H29N5O3 | 62 | 447.53 | 2.90 | 4 | 5 | 4 | 129.92 | 104.38 |
| 7b | C26H31N5O3 | 65 | 461.56 | 3.29 | 4 | 5 | 4 | 134.27 b | 104.38 |
| 7c | C26H31N5O4 | 66 | 477.56 | 2.74 | 4 | 9 | 4 | 136.38 b | 113.61 |
| 7d | C27H33N5O5 | 70 | 507.58 b | 2.58 | 4 | 7 | 3 b | 142.84 | 122.84 |
| 7e | C28H35N5O6 | 74 b | 537.61 | 2.43 | 4 | 8 | 3 | 149.31 | 132.07 |
| 7f | C26H29N5O5 | 65 | 491.54 | 2.52 | 4 | 7 | 4 | 135.68 b | 122.84 |
| 7g | C25H28ClN5O3 | 62 | 481.97 | 3.50 | 4 | 8 | 4 | 134.72 b | 104.38 |
| 7h | C25H28FN5O3 | 62 | 465.52 | 3.04 | 4 | 5 | 4 | 130.13 | 104.38 |
| 7i | C26H28F3N5O3 | 65 | 515.53 b | 3.78 | 4 | 5 | 3 b | 135.89 b | 104.38 |
| 7j | C26H28F3N5O3 | 65 | 515.53 b | 3.78 | 4 | 5 | 3 b | 135.89 b | 104.38 |
| 7k | C27H27F6N5O3 | 68 | 583.53 | 4.65 | 4 | 5 | 3 | 141.86 | 104.38 |
| Compd. | Structural Formula | Cell Line | |||||
|---|---|---|---|---|---|---|---|
| L1210 | CEM | HeLa | NCI-H460 | SW 620 | MCF-7 | ||
| 3a |  | 52 ± 3 | 27 ± 4 | 4.0 ± 0.9 | >1 | 23 ± 9 | 24 ± 5 |
| 3b |  | 51 ± 0 | 55 ± 6 | 106 ± 26 | >100 | >100 | 9.4 ± 0.2 |
| 3c |  | 106 ± 6 | 61 ± 30 | >125 | >100 | >100 | 20 ± 3 |
| 3d |  | 100 ± 34 | 90 ± 45 | >125 | >100 | >100 | 16 ± 0.6 |
| 3e |  | 22 ± 2 | 55 ± 16 | >125 | >100 | >100 | 8.7 ± 0.3 |
| 3f |  | 59 ± 2 | 37 ± 27 | 72 ± 57 | >100 | >100 | 6.9 ± 1.3 |
| 3g |  | 63 ± 2 | 20 ± 2 | 36 ± 16 | >100 | >100 | 4.3 ± 1.0 |
| 3h |  | 66 ± 5 | 18 ± 15 | 2.1 ± 2.1 | >100 | 0.3 ± 0.1 | 1.1 ± 0.6 |
| 3i |  | 68 ± 2 | 41 ± 15 | 112 ± 11 | >100 | 64 ± 41 | 11 ± 2 |
| 3j |  | 50 ± 18 | 14 ± 1 | 25 ± 6 | >100 | >100 | 3.9 ± 0.6 |
| 3k |  | 92 ± 24 | 68 ± 29 | 18 ± 1 | >100 | >100 | 2.6 ± 0.5 |
| 7a |  | 7.0 ± 3.1 | 3.0 ± 0.5 | 12 ± 2 | >100 | >100 | 2.5 ± 1.9 |
| 7b |  | 53 ± 5 | 25 ± 14 | 47 ± 6 | 50 ± 4 | 9.5 ± 0.9 | 16 ± 9 |
| 7c |  | 1.7 ± 0.4 | 1.3 ± 0.7 | 2.4 ± 0.2 | >100 | >100 | 0.4 ± 0.2 |
| 7d |  | 40 ± 14 | 93 ± 46 | 92 ± 30 | 32 ± 21 | 25 ± 9 | 1.9 ± 1.8 |
| 7e |  | 57 ± 5 | 54 ± 12 | 70 ± 2 | 63 ± 2 | 48 ± 17 | 1.4 ± 0.1 |
| 7f |  | 4.8 ± 0.2 | 7.4 ± 2.1 | 31 ± 0 | 27 ± 3 | 17 ± 5 | 0.6 ± 0.3 |
| 7g |  | 1.6 ± 0.7 | 0.9 ± 0.7 | 2.7 ± 1.2 | 20 ± 0.7 | 21 ± 4 | 0.2 ± 0.2 |
| 7h |  | 40 ± 4 | 15 ± 2 | 46 ± 2 | 12 ± 0.2 | 12 ± 4 | 5.9 ± 2.0 |
| 7i |  | 9.4 ± 4.8 | 10 ± 1 | 10 ± 1 | 18 ± 3 | 16 ± 5 | 2.3 ± 0.2 |
| 7j |  | 27 ± 0 | 9.4 ± 5.6 | 28 ± 12 | 34 ± 2 | 40 ± 5 | 0.03 ± 0.02 |
| 7k |  | 30 ± 17 | 17 ± 5 | 30 ± 23 | 48 ± 2 | 32 ± 4 | 3.2 ± 0.8 |
| PQ | – | – | – | 30 ± 7 | 20 ± 6 b | 28 ± 10 | |
| SOR | 4.2 ± 2.4 | 3.2 ± 1.7 | 7.1 ± 2.6 | 6.1 ± 0.6 c | 7.1 ± 1.9 | 3.9 ± 1.6 | |
| CIS | – | – | – | 1 ± 0.1 | 7 ± 2 b | 10 ± 1 | |
| 5-FU | 0.5 ± 0.2 | 18 ± 5 | 0.54 ± 0.1 | 3 ± 0.3 | 4 ± 0.7 b | 15 ± 2 | |
| Compd. | RA (%) a | RA (%) b | LOX Inhibition a (%) (IC50 μΜ) | LP Inhibition b (%) | ||
|---|---|---|---|---|---|---|
| 20 min | 60 min | 20 min | 60 min | |||
| 3a | 53 | 33 | 16 | 20 | 19 | 44 |
| 3b | 5 | 37 | 2 | 2 | 14 | 79 |
| 3c | 40 | 43 | 22 | 27 | 45 | 70 |
| 3d | 34 | 31 | na | na | 43 | 73 |
| 3e | 16 | 23 | 4 | 9 | 26 | 47 |
| 3f | 11 | 33 | na | na | 34 | 87 |
| 3g | 27 | 46 | na | na | 17 | 46 |
| 3h | na | na | na | na | 10 | 66 |
| 3i | na | 8 | na | 13 | 19 | 32 |
| 3j | 38 | 40 | na | na | (50) | 77 |
| 3k | na | 50 | na | na | 43 | 65 |
| 7a | 39 | 100 | 49 | 100 | 36 | 83 |
| 7b | 84 | 99 | 42 | 100 | (100) | 62 |
| 7c | 39 | 100 | 54 | 100 | (50) | 89 |
| 7d | 100 | 100 | 55 | 100 | (10) | 88 |
| 7e | 46 | 100 | 60 | 100 | (43) | 86 |
| 7f | 74 | 100 | 47 | 100 | (55) | 88 |
| 7g | 100 | 100 | 66 | 100 | (70) | 89 |
| 7h | 52 | 100 | 61 | 100 | (41) | 67 |
| 7i | 96 | 73 | 71 | 100 | (42.5) | 84 |
| 7j | 100 | 95 | 42 | 79 | (35) | 50 |
| 7k | 98 | 97 | 72 | 100 | (67.5) | 55 |
| NDGA | 89 | 94 | 83 | 87 | (5.5) | nt |
| Trolox | nt | nt | nt | nt | nt | 88 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavić, K.; Perković, I.; Gilja, P.; Kozlina, F.; Ester, K.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Pontiki, E.; Zorc, B. Design, Synthesis and Biological Evaluation of Novel Primaquine-Cinnamic Acid Conjugates of the Amide and Acylsemicarbazide Type. Molecules 2016, 21, 1629. https://doi.org/10.3390/molecules21121629
Pavić K, Perković I, Gilja P, Kozlina F, Ester K, Kralj M, Schols D, Hadjipavlou-Litina D, Pontiki E, Zorc B. Design, Synthesis and Biological Evaluation of Novel Primaquine-Cinnamic Acid Conjugates of the Amide and Acylsemicarbazide Type. Molecules. 2016; 21(12):1629. https://doi.org/10.3390/molecules21121629
Chicago/Turabian StylePavić, Kristina, Ivana Perković, Petra Gilja, Filip Kozlina, Katja Ester, Marijeta Kralj, Dominique Schols, Dimitra Hadjipavlou-Litina, Eleni Pontiki, and Branka Zorc. 2016. "Design, Synthesis and Biological Evaluation of Novel Primaquine-Cinnamic Acid Conjugates of the Amide and Acylsemicarbazide Type" Molecules 21, no. 12: 1629. https://doi.org/10.3390/molecules21121629






