Antifungal Activity of Oleuropein against Candida albicans—The In Vitro Study
Abstract
:1. Introduction
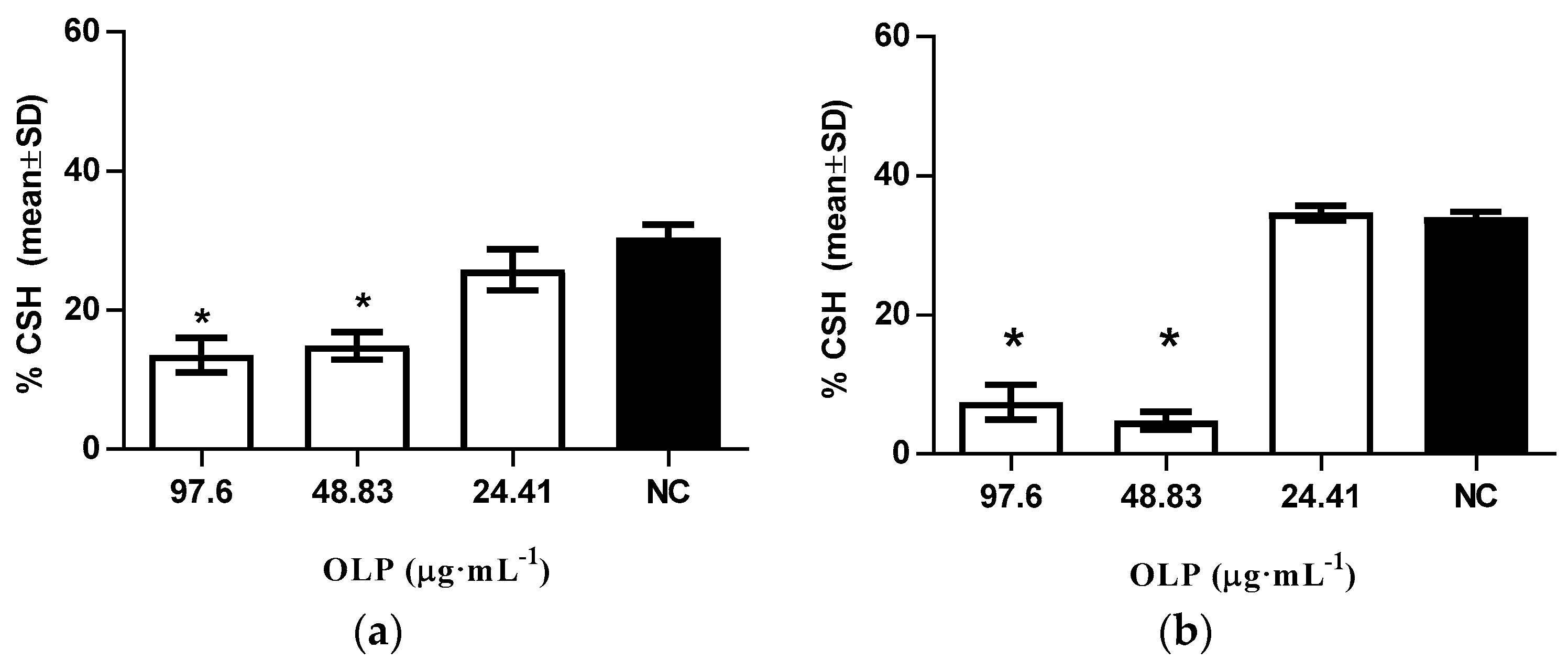
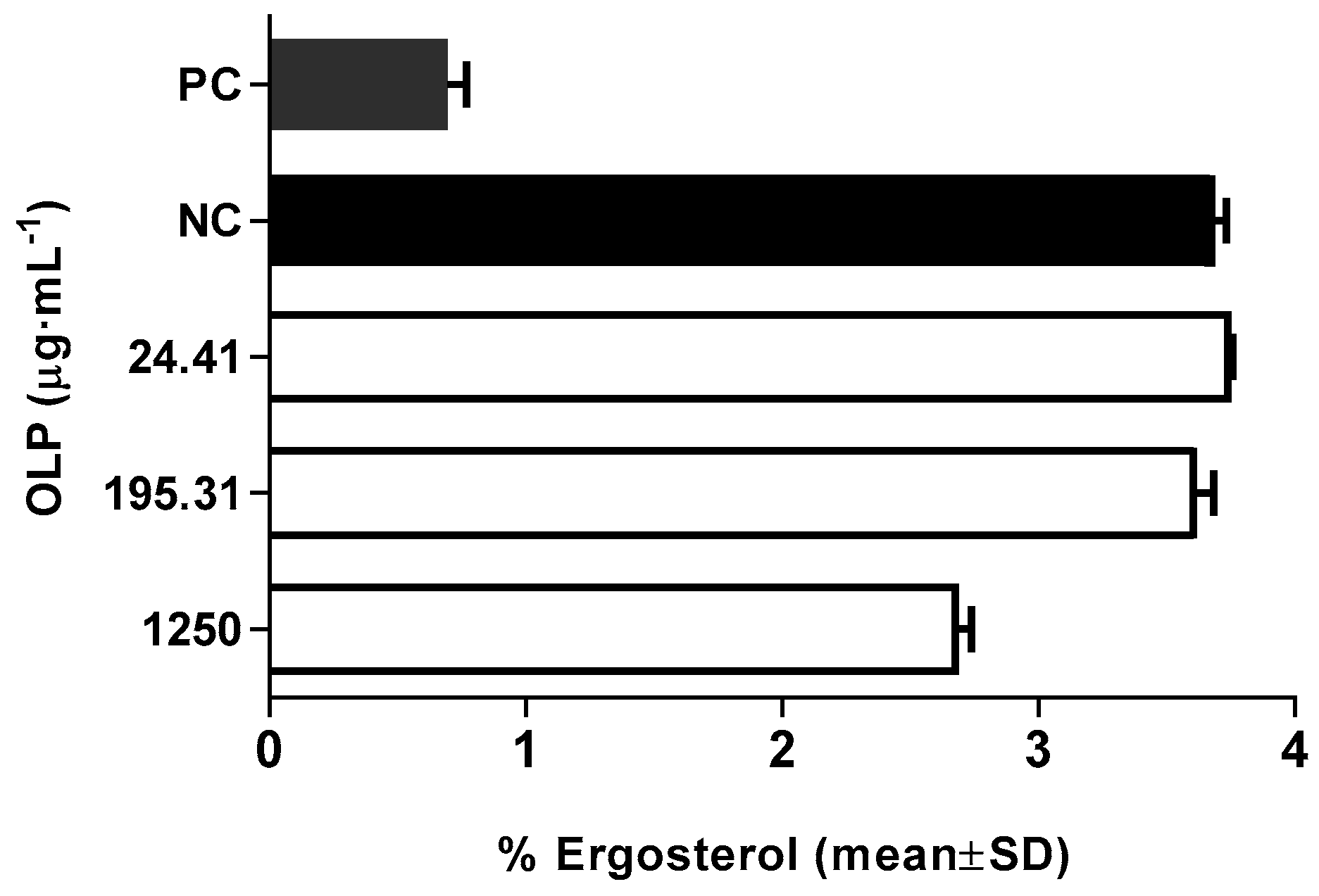
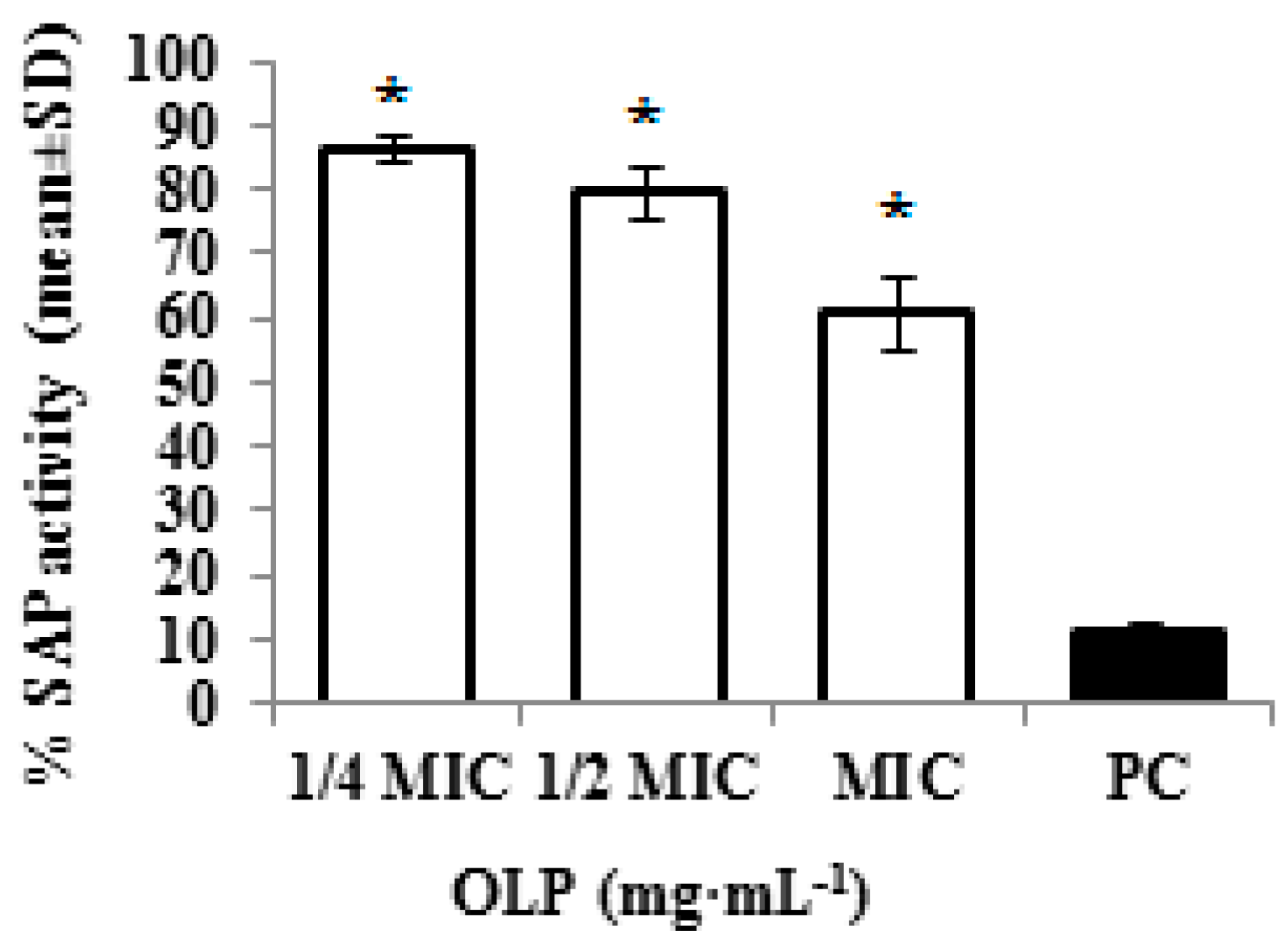
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Antimicrobial Susceptibility Testing
3.2.2. Identification of Apoptotic and Necrotic Cells Due to Loss of Membrane Integrity
3.2.3. Inhibition of Germ-Tube Formation
3.2.4. Modulation of Cellular Surface Hydrophobicity Levels
3.2.5. Modulation of Membrane Ergosterol Content
3.2.6. Inhibition of Secreted Aspartyl Proteinase Activity
4. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Zorić, N.; Horvat, I.; Kopjar, N.; Vučemilović, A.; Kremer, D.; Tomić, S.; Kosalec, I. Hydroxytyrosol expresses antifungal activity in vitro. Curr. Drug Targets 2013, 14, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Zorić, N.; Kopjar, N.; Oršolić, N.; Tomić, S.; Kosalec, I. Olive leaf extract activity against Candida albicans and C. dubliniensis—The in vitro viability study. Acta Pharm. 2016, 66, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.P.; Walter, W.M.; Etchells, J.L. Antimicrobial properties of oleuropein and products of its hydrolysis. J. Appl. Microbiol. 1973, 26, 777–782. [Google Scholar]
- Rodriguez, M.M.; Perez, J.; Ramos-Cormenzana, A.; Martinez, J. Effect of extracts obtained from olive oil mill waste waters on Bacillus megaterium ATCC 33085. J. Appl. Bacteriol. 1988, 64, 219–225. [Google Scholar] [CrossRef]
- Tassou, C.C.; Nychas, G.J.E.; Board, R.G. Effect of phenolic compounds and oleuropein on the germination of Bacillus cereus T spores. Biotechnol. Appl. Biochem. 1991, 13, 231–237. [Google Scholar] [PubMed]
- Furneri, P.M.; Marino, A.; Saija, A.; Uccella, N.; Bisignano, G. In vitro antimycoplasmal activity of oleuropein. Int. J. Antimicrob. Agents 2002, 20, 293–296. [Google Scholar] [CrossRef]
- Teodoro, G.; Ellepola, K.; Senevirante, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, J.A.; Samaranayake, Y.H.; Cheung, L.K.; Samaranayake, L.P. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J. Oral Pathol. Med. 2006, 35, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Midkiff, J.; Borochoff-Porte, N.; White, D.; Johnson, D. Small molecule inhibitors of the Candida albicans budded-to-hyphal transition act through multiple signaling pathways. PLoS ONE 2011, 6, e25395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Z.; Xu, J. Quantitative variation of biofilms among strains in natural population of Candida albicans. Microbiology 2003, 149, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Hazen, K.C.; Brawner, D.L.; Riesselman, M.H.; Jutila, M.A.; Cutler, J.E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect. Immun. 1991, 59, 907–912. [Google Scholar] [PubMed]
- Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Evolution of ergosterol inhibitors as fungicidal against Candida. Microb. Pathog. 2010, 48, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2011, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.R.; Jesuino, R.S.A.; Lemos, J.A.; Fernandes, O.F.L.; Souza, L.K.H.; Passos, X.S.; Silva, M.R.R. Effects of antifungal agents in Sap activity of Candida albicans isolates. Mycopathologia 2010, 169, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Stehr, F.; Felk, A.; Kretschmar, M.; Schaller, M.; Schäfer, W.; Hube, B. Extracellular hydrolytic enzymes and their relevance during Candida albicans infections. Mycoses 2000, 2, 17–21. [Google Scholar]
- EUCAST Document E.DEF. 7.3: Method for the Determination of Broth Dilution of Antifungal Agents for Fermentative Yeasts. Available online: http://www.eucast.org/ast_of_fungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts (accessed on 15 October 2015).
- Kosalec, I.; Puel, O.; Delaforge, M.; Kopjar, N.; Antolović, R.; Jelić, D.; Matica, B.; Galtier, P.; Pepeljnjak, S. Isolation and cytotoxicity of low-molecular-weight metabolites of Candida albicans. Front. Biosci. 2008, 13, 6893–6904. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Hér. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Palazzo de Mello, J.C.; Cortez, D.A.G.; Filho, B.P.D.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [PubMed]
- Yordanov, M.; Dimitrova, P.; Patkar, S.; Saso, L.; Ivanovska, N. Inhibition of Candida albicans extracellular enzyme activity by selected natural substances and their application in Candida infection. Can. J. Microbiol. 2008, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.


| Sample | Viable Cells (%) | Non-Viable Cells | ||
|---|---|---|---|---|
| Σ | Apoptosis (%) | Necrosis (%) | ||
| OLP1 | 16.3 ± 2.1 | 83.7 ± 2.1 OLP2,OLP3,NC | 39.3 ± 11.2 OLP2,OLP3,NC,PC | 44.3 ± 10.2 OLP2,OLP3,NC,PC |
| OLP2 | 61.3 ± 6.0 | 38.7 ± 6.0 OLP3,NC,PC | 29.0 ± 4.6 OLP3,NC,PC | 9.7 ± 1.5 NC |
| OLP3 | 76.0 ± 6.6 | 24.0 ± 6.6 NC,PC | 14.7 ± 5.5 NC,PC | 9.3 ± 1.5 NC |
| PC | 11.7 ± 3.2 | 88.3 ± 3.2 NC | 78.7 ± 0.6 NC | 9.7 ± 3.8 NC |
| NC | 97.3 ± 0.6 | 2.7 ± 0.6 | 1.0 ± 1.0 | 1.7 ± 1.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorić, N.; Kopjar, N.; Bobnjarić, I.; Horvat, I.; Tomić, S.; Kosalec, I. Antifungal Activity of Oleuropein against Candida albicans—The In Vitro Study. Molecules 2016, 21, 1631. https://doi.org/10.3390/molecules21121631
Zorić N, Kopjar N, Bobnjarić I, Horvat I, Tomić S, Kosalec I. Antifungal Activity of Oleuropein against Candida albicans—The In Vitro Study. Molecules. 2016; 21(12):1631. https://doi.org/10.3390/molecules21121631
Chicago/Turabian StyleZorić, Nataša, Nevenka Kopjar, Ivan Bobnjarić, Igor Horvat, Siniša Tomić, and Ivan Kosalec. 2016. "Antifungal Activity of Oleuropein against Candida albicans—The In Vitro Study" Molecules 21, no. 12: 1631. https://doi.org/10.3390/molecules21121631







