A Novel Two-Step Liquid-Liquid Extraction Procedure Combined with Stationary Phase Immobilized Human Serum Albumin for the Chiral Separation of Cetirizine Enantiomers along with M and P Parabens
Abstract
:1. Introduction
2. Results and Discussion
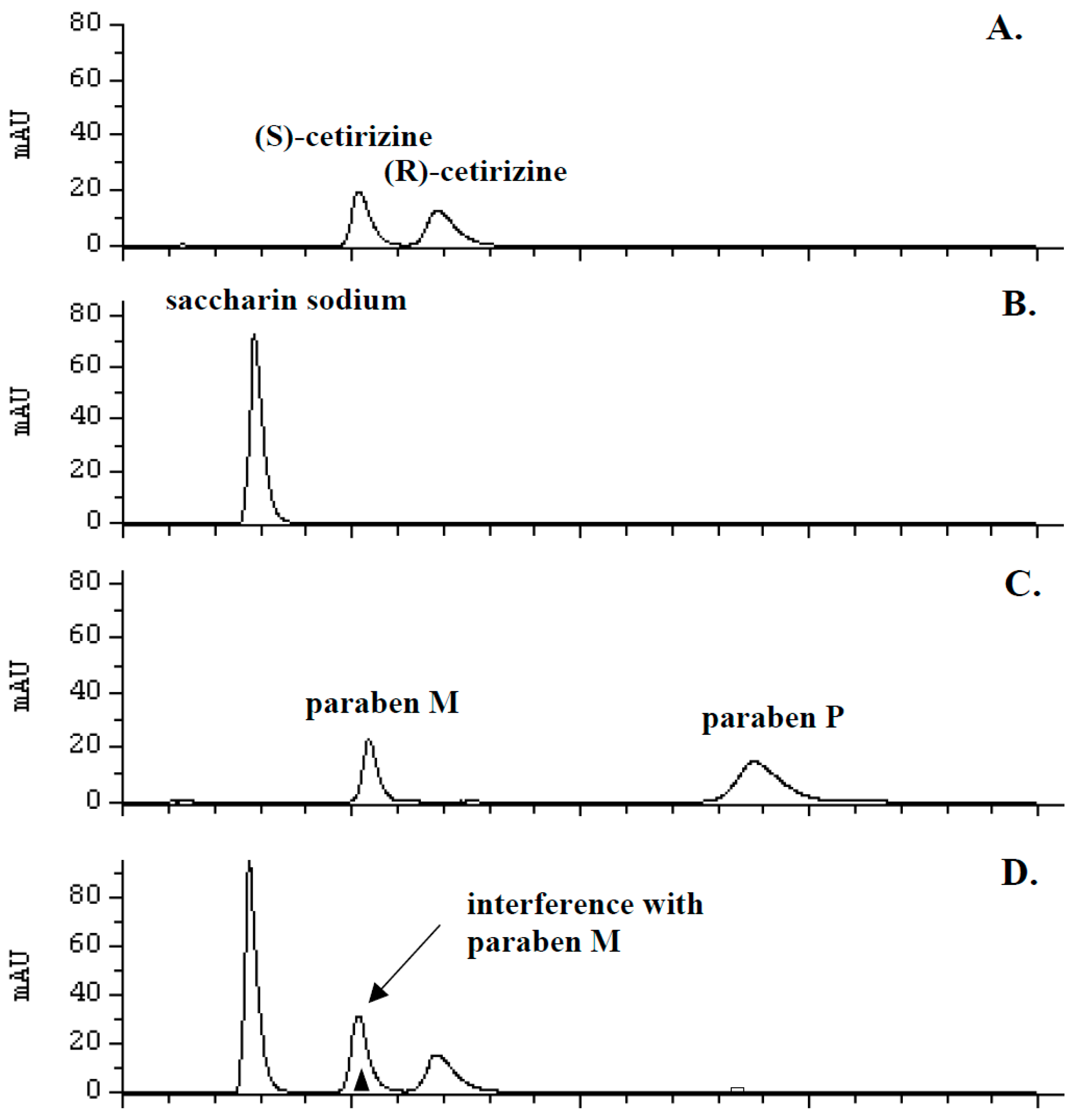
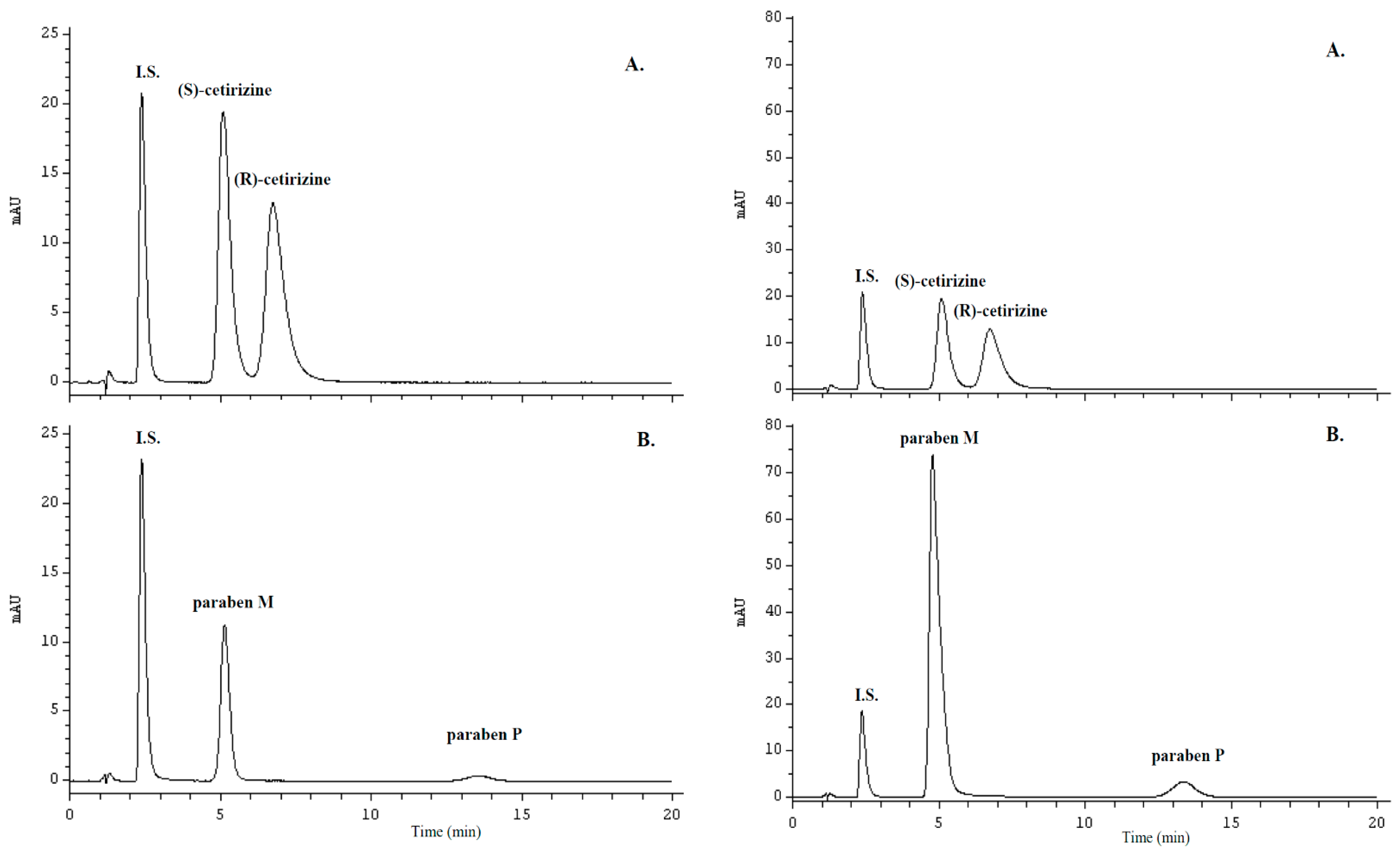
2.1. Chromatographic Conditions and Selectivity
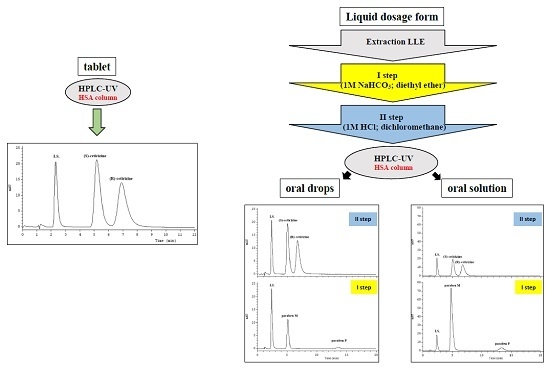
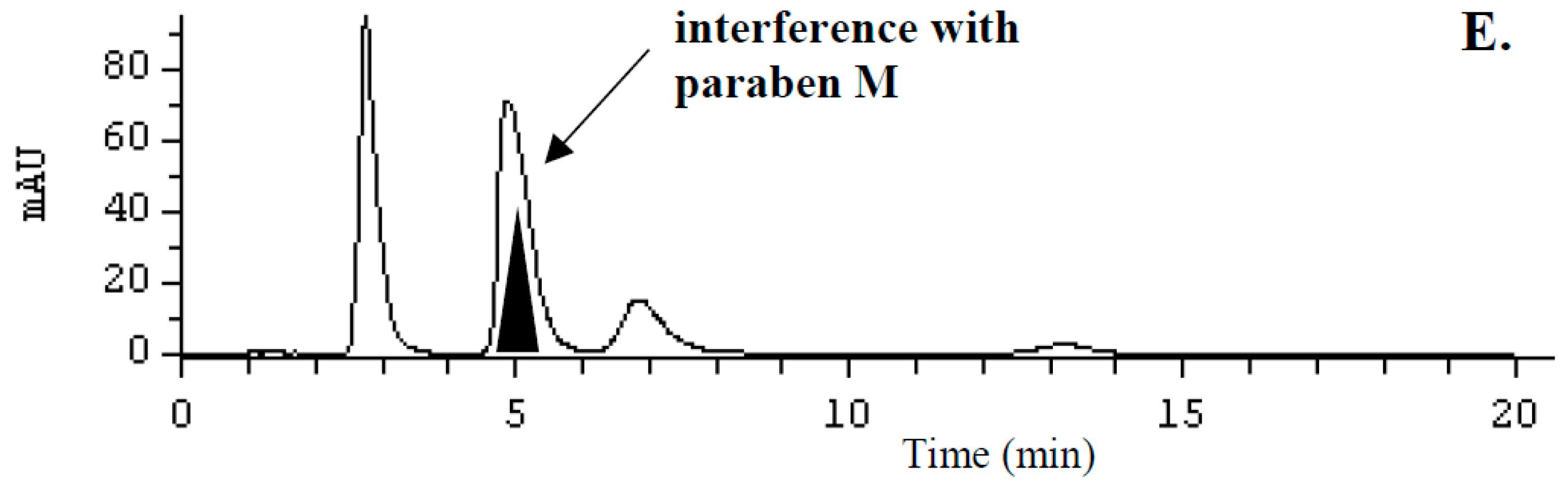
2.2. Optimization of Sample Preparation
2.3. Response Function and Linearity
2.4. Precision and Accuracy
2.5. Application of the Validated Method to Pharmaceutical Products
3. Experimental Section
3.1. Reagents
3.2. Instrumentation
3.3. Preparation of Stock and Standard Solutions
3.4. Sample Preparation
3.4.1. Tablets
3.4.2. Drops and Oral Solution
3.5. Chromatographic Conditions
3.6. Validation of the Analytical Method
3.6.1. Linearity
3.6.2. Sensitivity
3.6.3. Precision
3.6.4. Accuracy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tillement, J.-P.; Testa, B.; Brée, F. Compared pharmacological characteristics inhumans of racemic cetirizine and levocetirizine, two histamine H1-receptor antagonists. Biochem. Pharmacol. 2003, 66, 1123–1126. [Google Scholar] [CrossRef]
- Bajerski, L.; da Silva Sangoi, M.; Barth, T.; Diefenbach, I.F.; Dalmora, S.L.; Cardoso, S.G. Determination of cetirizine in tablets and compounded capsules: Comparative study between Ce and HPLC. Quim. Nova 2010, 33, 114–118. [Google Scholar] [CrossRef]
- Paw, B.; Misztal, G.; Hopkała, H.; Drozd, J. Development and validation of a HPLC method for the determination of cetirizine in pharmaceutical dosage forms. Pharmazie 2002, 57, 313–315. [Google Scholar] [PubMed]
- Arayne, M.S.; Sultana, N.; Siddiqui, F.A. Determination and quantification of cetirizine HCl in dosage formulations by RP-HPLC. Pak. J. Pharm. Sci. 2005, 18, 7–11. [Google Scholar] [PubMed]
- Jaber, A.M.Y.; Al Sherife, H.A.; Al Omari, M.M.; Badwan, A.A. Determination of cetirizine dihydrochloride, related impurities and preservatives in oral solution and tablet dosage forms using HPLC. J. Pharm. Biomed. Anal. 2004, 36, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Uysal, Ü.D.; Tunçel, M. Validated capillary electrophoresis study for the determination of cetirizine in pharmaceutical forms. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1781–1792. [Google Scholar] [CrossRef]
- Mikuš, P.; Valášková, I.; Havránek, E. Enantioselective analysis of cetirizine in pharmaceuticals by cyclodextrin-mediated capillary electrophoresis. J. Sep. Sci. 2005, 28, 1278–1284. [Google Scholar]
- Greige-Gerges, H.; Kaissi, R.; Magdalou, J.; Jraij, A. Reviewing the binding of a series of parabens to human serum albumin. Biopharm. Drug Dispos. 2013, 34, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.O.; Lee, S.H.; Kong, H.S.; Kim, E.J.; Choo, H.Y.P. Stereoselective determination of cetirizine and studies on pharmacokinetics in rat plasma. J. Chromatogr. B 2000, 744, 201–206. [Google Scholar] [CrossRef]
- Choi, S.O.; Lee, S.H.; Kong, H.S.; Kim, E.J.; Choo, H.Y.P. Enantioselective determination of cetirizine in human urine by HPLC. Arch. Pharm. Res. 2000, 23, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jansson, B.; Chatelain, P.; Massingham, R.; Hammarlund Udenaes, M. Quantitative determination of cetirizine enantiomers in guinea pig plasma, brain tissue and microdialysis samples using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Yoo, S.D. Simultaneous determination of levocetirizine and pseudoephedrine in dog plasma by liquid chromatography-mass spectrometry in the presence of dextrocetirizine. J. Pharm. Pharm. Sci. 2012, 15, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Z.; Bo, H.; Sheldon, R.A. Direct separation of the enantiomers of cetirizine and related compounds by reversed-phase chiral HPLC. Chromatographia 2002, 56, 233–235. [Google Scholar] [CrossRef]
- Kang, S.W.; Jang, H.J.; Moore, V.S.; Park, J.-Y.; Kim, K.-A.; Youm, J.-R.; Han, S.B. Enantioselective determination of cetirizine in human plasma by normal-phase liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Cristofol, C.; Alonso, C. Study of the enantiomeric separation of oxfendazole and cetirizine using subcritical fluid chromatography on an amylose-based column. J. Chromatogr. A 2006, 1121, 268–273. [Google Scholar] [CrossRef] [PubMed]
- De Klerck, K.; Vander Heyden, Y.; Mangelings, D. Generic chiral method development in supercritical fluid chromatography and ultra-performance supercritical fluid chromatography. J. Chromatogr. A 2014, 1363, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.Y.; Kang, M.; Kang, S.W.; Kim, U.; Suh, J.H.; Kim, J.; Cho, H.-D.; Jung, Y.; Yang, D.-H.; Han, S.B. Rapid chiral separation of racemic cetirizine in human plasma using subcritical fluid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 117, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.W.; Huang, W.S.; Ko, C.C.; Chen, S.H. Enantioseparation of cetirizine by sulfated-β-cyclodextrin-mediated capillary electrophoresis. J. Sep. Sci. 2008, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Van Eeckhaut, A.; Michotte, Y. Chiral separation of cetirizine by capillary electrophoresis. Electrophoresis 2006, 27, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; de Cock, B.; Vervoort, R.; Pamperin, D.; Adams, E.; van Schepdael, A. Development of a validated capillary electrophoresis method for enantiomeric purity control and quality control of levocetirizine in a pharmaceutical formulation. Chirality 2012, 24, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Du, Y.X.; Zhu, F.X.; Chen, B. Glycogen: A novel branched polysaccharide chiral selector in CE. Electrophoresis 2010, 31, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Guo, B.Y.; Lin, J.M. Ultra-high concentration of amylose for chiral separations in capillary electrophoresis. J. Chromatogr. A 2009, 1216, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Tabani, H.; Fakhari, A.R.; Nojavan, S. Maltodextrins as chiral selectors in CE: Molecular structure effect of basic chiral compounds on the enantioseparation. Chirality 2014, 26, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Du, Y. Evaluation of the enantioseparation capability of the novel chiral selector clindamycin phosphate towards basic drugs by micellar electrokinetic chromatography. J. Chromatogr. A 2010, 1217, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Le Ngoc, T.; Shuchi, D.; Jung, H.P. Enantioseparation of basic chiral compounds on a clindamycin phosphate-silica/zirconia hybrid monolith by capillary electrochromatography. J. Chromatogr. A 2014, 1356, 289–293. [Google Scholar]
- Avvaru, P.K.; Jung, H.P. Azithromycin as a new chiral selector in capillary electrophoresis. J. Chromatogr. A 2011, 1218, 1314–1317. [Google Scholar]
- Basavaiah, K.; SriLatha; Swamy, J.M. Spectrophotometric determination of cetirizine hydrochloride with Alizarin Red S. Talanta 1999, 50, 887–892. [Google Scholar] [CrossRef]
- Gowda, B.G.; Melwanki, M.B.; Seetharamappa, J. Extractive spectrophotometric determination of cetirizine HCl in pharmaceutical preparations. J. Pharm. Biomed. Anal. 2001, 25, 1021–1026. [Google Scholar] [CrossRef]
- Drozd, J.; Hopkała, H.; Misztal, G.; Paw, B. Applications of derivative UV-spectrophotometry for the determinations of cetirizine dihydrochloride in pharmaceutical preparations. Acta Pol. Pharm. 2002, 59, 3–7. [Google Scholar] [PubMed]
- British Pharmacopoeia 2012; The Stationery Office: London, UK, 2012; pp. 2572–2573.
- Kowalski, P.; Plenis, A. Comparison of HPLC and CE methods for the determination of cetirizine dihydrochloride in human plasma samples. Biomed. Chromatogr. 2007, 21, 903–911. [Google Scholar]
- International Conferences on Harmonization (ICH). ICH Harmonized Tripartite Guideline Validation of Analytical Procedures: Text and Methodology, Q2 (R1). ed.; International Conferences on Harmonization: Geneva, Switzerland, 2005. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.

| Mobile Phase | Retention Factor k | Enantioselectivity Factor α | Resolution Factor Rs | |||||
|---|---|---|---|---|---|---|---|---|
| Organic Solvent (A) | PB a mM pH 7 (B) | A:B (v/v) | Flow-Rate (mL·min−1) | Temp. (°C) | PM b | (S)-Cetirizine | ||
| 2-propanol | 10 | 14:86 | 0.9 | 25 | 2.72 | 2.65 | 1.10 | 0.80 |
| 2-propanol | 10 | 10:90 | 0.9 | 25 | 3.27 | 3.23 | 1.43 | 1.82 |
| 0.7 | 25 | 4.85 | 4.75 | 1.32 | 1.62 | |||
| 0.5 | 25 | 5.78 | 5.85 | 1.20 | 1.40 | |||
| 0.9 | 18 | 5.10 | 4.98 | 1.30 | 1.45 | |||
| 2-propanol | 10 | 6:94 | 0.9 | 25 | 5.75 | 5.82 | 1.21 | 1.54 |
| 2-propanol | 20 | 6:94 | 0.9 | 25 | 5.65 | 5.61 | 1.25 | 1.59 |
| acetonitrile | 10 | 10:90 | 0.9 | 25 | 3.05 | 3.10 | 1.15 | 0.65 |
| acetonitrile | 10 | 6:94 | 0.9 | 25 | 3.82 | 3.90 | 1.26 | 1.12 |
| acetonitrile | 20 | 6:94 | 0.9 | 25 | 3.78 | 3.77 | 1.25 | 1.10 |
| acetonitrile | 10 | 5:94 | 25 | 5.80 | 5.90 | 1.38 | 1.65 | |
| 0.9 | 18 | 7.45 | 7.54 | 1.28 | 1.38 | |||
| acetonitrile | 10 | 4:96 | 0.9 | 25 | 6.88 | 6.75 | 1.32 | 1.45 |
| acetonitrile | 20 | 4:96 | 0.9 | 25 | 6.70 | 6.62 | 1.38 | 1.48 |
| Tablets | Oral Drops or Oral Solution | |||||
|---|---|---|---|---|---|---|
| Parameter | (S)-Cetirizine | (R)-Cetirizine | (S)-Cetirizine | (R)-Cetirizine | Paraben M | Paraben P |
| Linearity range, µg·mL−1 | 0.25–25 | 0.25–25 | 0.25–25 | 0.25–25 | 0.45–54 | 0.05–6 |
| LOD, µg·mL−1 (a) | 0.1 | 0.1 | 0.1 | 0.1 | 0.02 | 0.02 |
| LOQ, µg·mL−1 (b) | 0.25 | 0.25 | 0.25 | 0.25 | 0.05 | 0.05 |
| Slope (±SD) | 0.207 (±0.002) | 0.207 (±0.002) | 0.204 (±0.003) | 0.205 (±0.003) | 0.190 (±0.002) | 0.166 (±0.002) |
| Intercept (±SD) | 0.031 (±0.023) | 0.032 (±0.025) | 0.024 (±0.036) | 0.023 (±0.034) | 0.006 (±0.047) | 0.0008 (±0.005) |
| Correlation coefficient, R (R2) | 0.9996 (0.9992) | 0.9996 (0.9991) | 0.9990 (0.9981) | 0.9991 (0.9983) | 0.9997 (0.9993) | 0.9995 (0.9991) |
| Standard error of the regression (sy/x) | 0.05 | 0.05 | 0.08 | 0.07 | 0.10 | 0.01 |
| Number of data points (n) | 11 | 11 | 11 | 11 | 10 | 10 |
| Nominal Concentration (μg·mL−1) | Intra-Day Precision (n = 6) | Inter-Day Precision (n = 6) | Accuracy as Recovery (%) | ||
|---|---|---|---|---|---|
| Concentration Found (±SD) (μg·mL−1) | RSD (%) | Concentration Found (±SD) (μg·mL−1) | RSD (%) | ||
| for direct determination in tablets | |||||
| (S)-cetirizine | |||||
| 2.5 | 2.50 ± 0.16 | 6.63 | 2.54 ± 0.18 | 7.15 | 99.84 |
| 10 | 10.00 ± 0.33 | 3.30 | 10.25 ± 0.37 | 3.58 | 100.00 |
| 20 | 20.25 ± 0.25 | 1.24 | 20.52 ± 0.30 | 1.35 | 101.23 |
| (R)-cetirizine | |||||
| 2.5 | 2.49 ± 0.16 | 6.58 | 2.53 ± 0.18 | 7.18 | 99.72 |
| 10 | 10.00 ± 0.32 | 3.25 | 10.25 ± 0.37 | 3.55 | 100.01 |
| 20 | 20.25 ± 0.25 | 1.23 | 20.53 ± 0.29 | 1.33 | 101.25 |
| for determination after two-step liquid-liquid extraction from oral drops or oral solution | |||||
| (S)-cetirizine | |||||
| 2.5 | 2.49 ± 0.17 | 6.79 | 2.53 ± 0.19 | 7.05 | 99.59 |
| 10 | 9.72 ± 0.34 | 3.48 | 9.83 ± 0.36 | 3.52 | 97.16 |
| 20 | 20.07 ± 0.27 | 1.32 | 20.10 ± 0.30 | 1.24 | 100.37 |
| (R)-cetirizine | |||||
| 2.5 | 2.49 ± 0.17 | 6.74 | 2.52 ± 0.19 | 6.96 | 99.69 |
| 10 | 9.75 ± 0.34 | 3.46 | 9.83 ± 0.37 | 3.55 | 97.47 |
| 20 | 20.10 ± 0.27 | 1.30 | 20.11 ± 0.29 | 1.25 | 100.48 |
| Paraben M | |||||
| 2.7 | 2.63 ± 0.19 | 7.23 | 2.68 ± 0.20 | 7.58 | 97.58 |
| 27 | 26.50 ± 0.81 | 3.06 | 27.22 ± 0.92 | 3.42 | 98.15 |
| 45 | 44.37 ± 0.68 | 1.53 | 44.83 ± 0.79 | 1.42 | 98.60 |
| Paraben P | |||||
| 0.3 | 0.29 ± 0.02 | 7.38 | 0.32 ± 0.03 | 7.68 | 97.07 |
| 3.0 | 3.00 ± 0.10 | 3.37 | 3.11 ± 0.14 | 3.56 | 99.92 |
| 5.0 | 5.04 ± 0.08 | 1.60 | 5.09 ± 0.10 | 1.50 | 100.86 |
| Pharmaceutical Preparation x | Label Claim | Recovery ± SD (%) y | |||
|---|---|---|---|---|---|
| (S)-Cetirizine | (R)-Cetirizine | Paraben M | Paraben P | ||
| ZYRTEC® coated tablets | 10 mg per tablet | 99.80 ± 0.53 | 99.83 ± 0.55 | n.d. z | n.d. z |
| ZYRTEC® drops | 10 mg·mL−1 | 98.65 ± 0.81 | 98.68 ± 0.83 | 98.52 ± 0.74 | 98.00 ± 0.85 |
| ZYRTEC® oral solution | 1 mg·mL−1 | 98.00 ± 0.60 | 98.10 ± 0.64 | 99.26 ± 0.85 | 98.77 ± 0.82 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewska, A.; Konieczna, L.; Bączek, T. A Novel Two-Step Liquid-Liquid Extraction Procedure Combined with Stationary Phase Immobilized Human Serum Albumin for the Chiral Separation of Cetirizine Enantiomers along with M and P Parabens. Molecules 2016, 21, 1654. https://doi.org/10.3390/molecules21121654
Chmielewska A, Konieczna L, Bączek T. A Novel Two-Step Liquid-Liquid Extraction Procedure Combined with Stationary Phase Immobilized Human Serum Albumin for the Chiral Separation of Cetirizine Enantiomers along with M and P Parabens. Molecules. 2016; 21(12):1654. https://doi.org/10.3390/molecules21121654
Chicago/Turabian StyleChmielewska, Aleksandra, Lucyna Konieczna, and Tomasz Bączek. 2016. "A Novel Two-Step Liquid-Liquid Extraction Procedure Combined with Stationary Phase Immobilized Human Serum Albumin for the Chiral Separation of Cetirizine Enantiomers along with M and P Parabens" Molecules 21, no. 12: 1654. https://doi.org/10.3390/molecules21121654