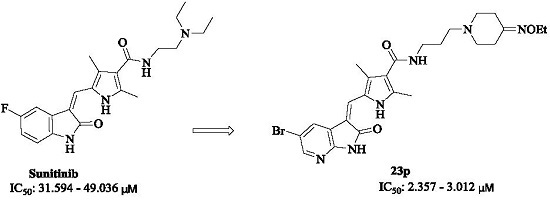
Synthesis and Antitumor Activity of 5-Bromo-7-azaindolin-2-one Derivatives Containing a 2,4-Dimethyl-1H-pyrrole-3-carboxamide Moiety
Abstract
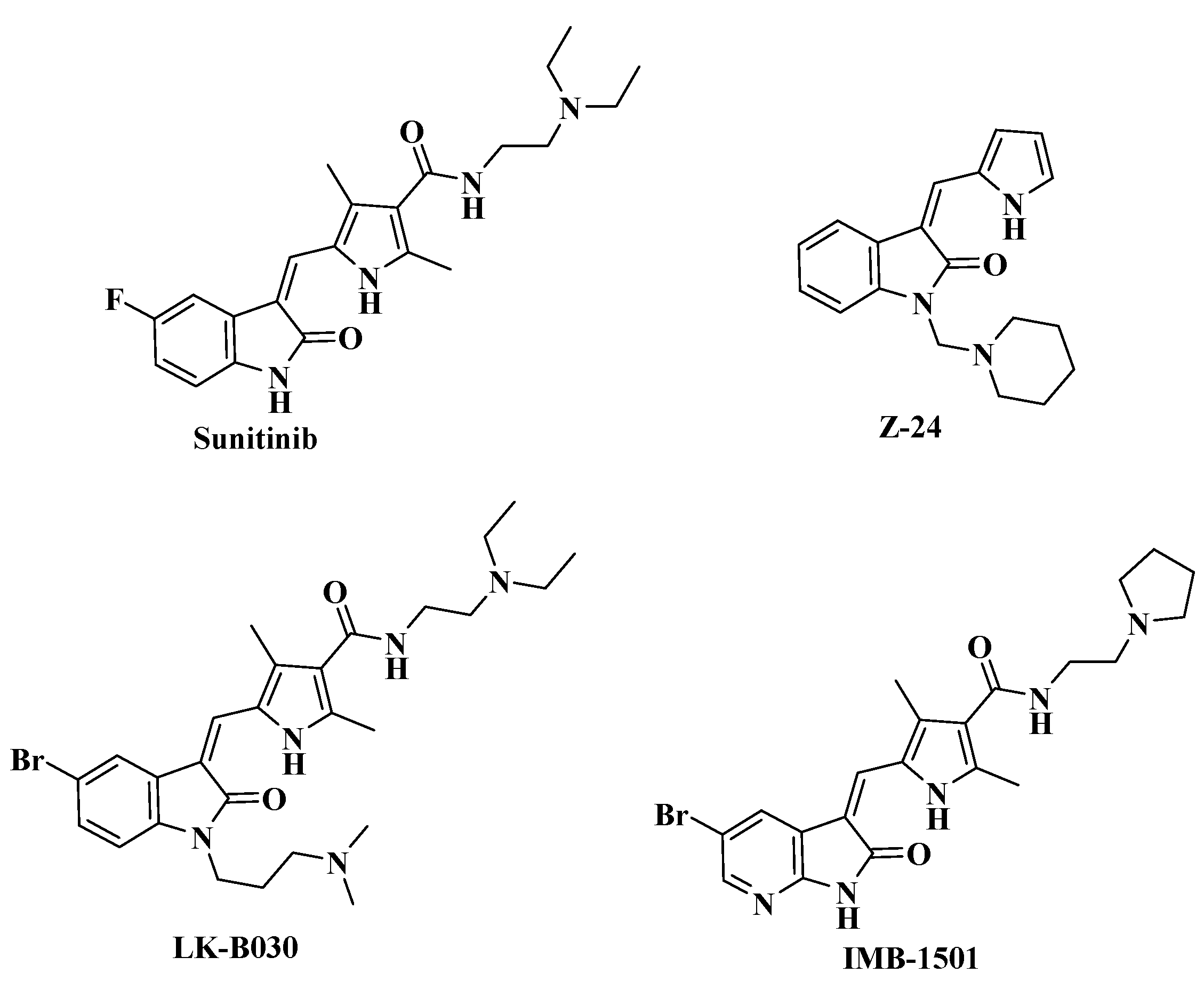
:1. Introduction
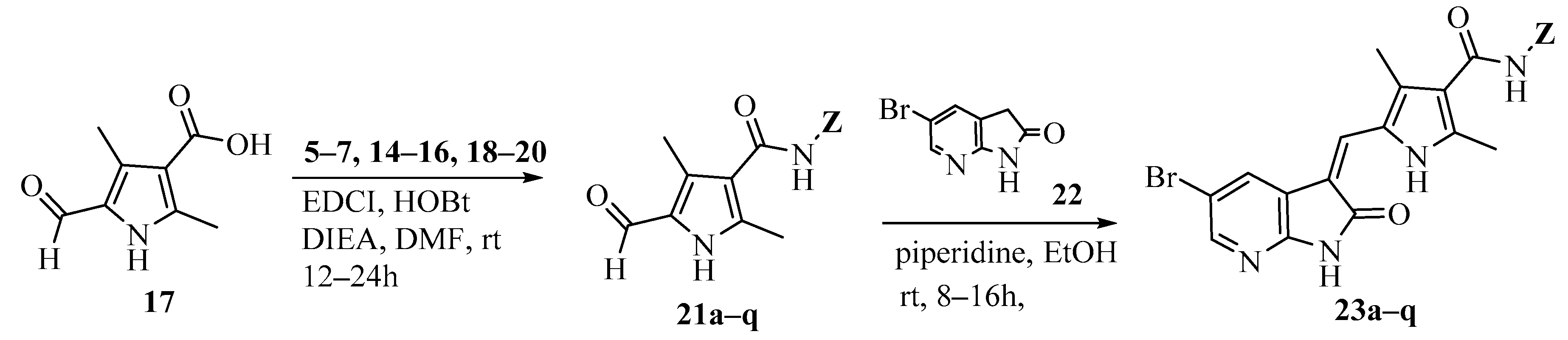
2. Results and Discussion
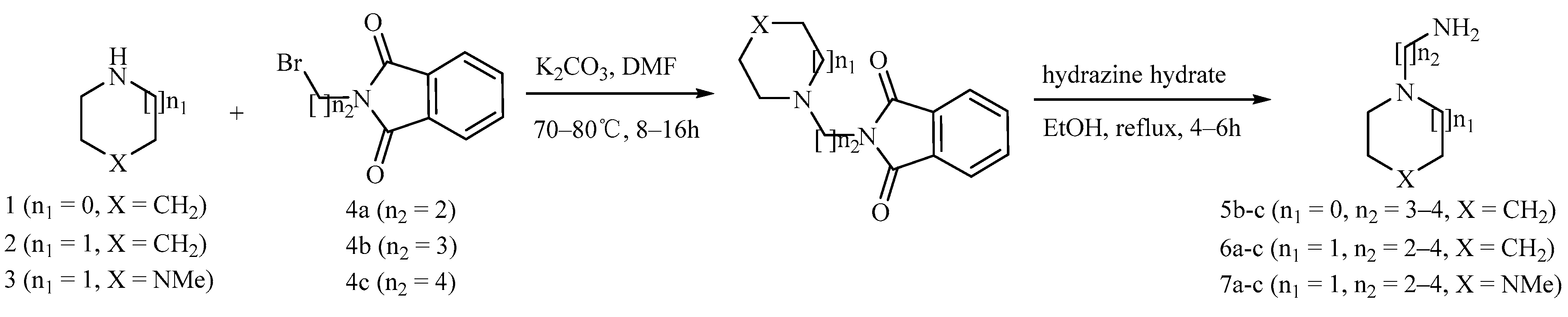
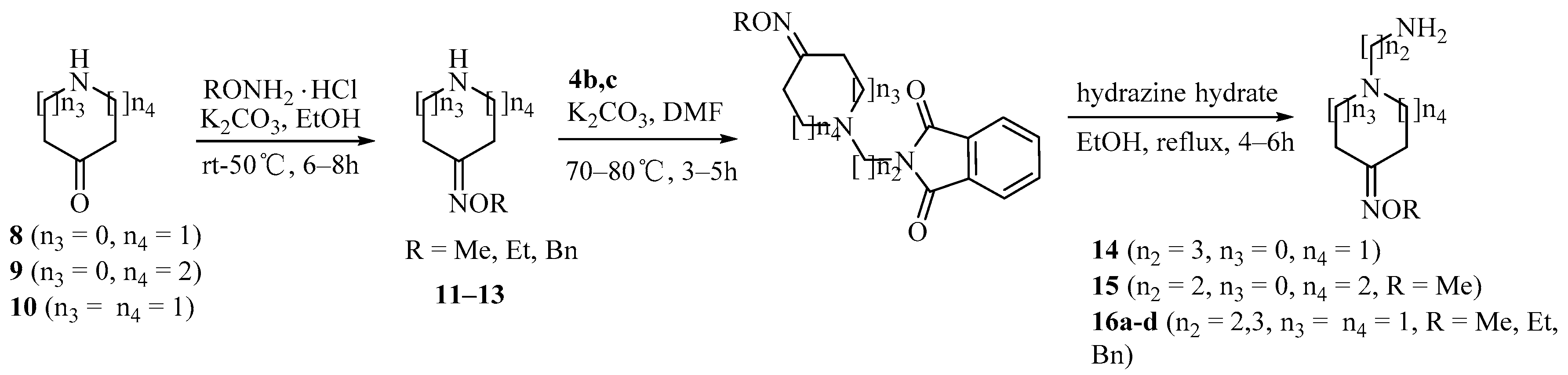
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kassem, M.G.; Motiur Rahman, A.F.M.; Korashy, H.M. Sunitinib malate. Profiles Drug Subst. Excip. Relat. Methodol. 2012, 37, 363–388. [Google Scholar] [PubMed]
- Kulke, M.H.; Bendell, J.; Kvols, L.; Picus, J.; Pommier, R.; Yao, J. Evolving Diagnostic and Treatment Strategies for Pancreatic Neuroendocrine Tumors. J. Hematol. Oncol. 2011, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Drappatz, J.; Wen, P.Y. Targeted drug therapy for meningiomas. Neurosurg. Focus 2007, 23, E12. [Google Scholar] [CrossRef] [PubMed]
- Houk, B.E.; Bello, C.L.; Poland, B.; Rosen, L.S.; Demetri, G.D.; Motzer, R.J. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: Results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother. Pharmacol. 2010, 66, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Scagliotti, G.V.; Rosell, R.; Socinski, M.A.; Brahmer, J.; Atkins, J.; Pallares, C.; Burgess, R.; Tye, L.; Selaru, P.; et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br. J. Cancer 2009, 101, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.; Bryant, J.; Chou, Y.L.; Kochanny, M.J.; Lee, W.; Phillips, G.B.; Yu, H.Y.; Adler, M.; Whitlow, M.; Ho, E.; et al. Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 1: Design, synthesis and biological activity. Bioorg. Med. Chem. Lett. 2007, 17, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.I.; Brown, G.; Bryant, J.; Hrvatin, P.; Kochanny, M.J.; Phillips, G.B.; Yuan, S.D.; Adler, M.; Whitlow, M.; Lentz, D.; et al. Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 2: Optimization of BX-517. Bioorg. Med. Chem. Lett. 2007, 17, 3819–3825. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Su, Y.D.; Feng, J.; Fu, J.H.; Yang, J.L.; Xiao, L.; Peng, J.H.; Li, Y.L.; Zhang, L.; Hu, B.; et al. Novel potent orally active multitargeted receptor tyrosine kinase inhibitors: Synthesis, structure-activity relationships, and antitumor activities of 2-indolinone derivatives. J. Med. Chem. 2010, 53, 8140–8149. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Lin, C.; Zheng, Z.B.; Li, S.; Guo, S.X.; Zhang, X.Y.; Guo, S.; Zhang, X.Y.; Fu, M.; Liang, X.; et al. Angiogenesis Inhibitor Z24 Induces Endothelial Cell Apoptosis and Suppresses Tumor Growth and Metastasis. J. Pharmacol. Sci. 2005, 97, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, C.; Shirazian, S.; Zhou, Y.; Miller, T.; Cui, J.; Fukuda, J.Y.; Chu, J.Y.; Nematalla, A.; Wang, X.Y.; et al. Discovery of 5-[5-Fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic Acid (2-Diethylaminoethyl)amide, a Novel Tyrosine Kinase Inhibitor Targeting Vascular Endothelial and Platelet-Derived Growth Factor Receptor Tyrosine Kinase. J. Med. Chem. 2003, 46, 1116–1119. [Google Scholar] [PubMed]
- Manley, J.M.; Kalman, M.J.; Conway, B.G.; Ball, C.C.; Havens, J.L.; Vaidyannathan, R. Early amidation approach to 3-[(4-amido)pyrrol-2-yl]-2-indolinones. J. Med. Chem. 2003, 68, 6447–6450. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Wang, L.L.; Liu, M.L.; Zhou, X.B.; Fan, S.Y.; Liu, H.Y.; Zheng, Z.B.; Li, S. Synthesis and antitumor activity of 5-[1-(3-(dimethylamino)propyl)-5-halogenated-2-oxoindolin-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2011, 21, 3062–3065. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Wang, L.L.; Zhou, X.B.; Liu, M.L.; Liu, H.Y.; Zheng, Z.B.; Li, S. Synthesis and in vitro antitumor activity of 1-(3-dimethylamino)propyl indolin-2-one derivatives. Med. Chem. Res. 2013, 22, 1723–1729. [Google Scholar] [CrossRef]
- Wang, M.; Ye, C.; Liu, M.L.; Wu, Z.Y.; Li, L.H.; Wang, C.L.; Liu, X.J.; Guo, H.Y. Synthesis and antitumor activity of 5-(5-halogenated-2-oxo-1H-pyrrolo[2,3-b]pyridin-(3Z)-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2015, 25, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, B.X.; Wu, T.; Wan, J.T.; Tu, Z.C.; Njire, M.; Wan, B.J.; Franzblauc, S.G.; Zhang, T.Y.; Lu, X.Y.; et al. Design, Synthesis, and Biological Evaluation of Pyrazolo[1,5-a]pyridine-3-carboxamides as Novel Antitubercular Agents. ACS Med. Chem. Lett. 2015, 6, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Green, L.M.; Reade, J.L.; Ware, C.F. Rapid colormetric assay for cell viability: Application to the quantitation of cytotoxic and growth inhibitory lymphokines. J. Immunol. Methods 1984, 70, 257–268. [Google Scholar] [CrossRef]
- Lv, K.; Sun, Y.X.; Sun, L.Y.; Wei, Z.Q.; Guo, H.Y.; Wu, J.W.; Liu, H.Y. Design, Synthesis, and in vitro Antibacterial Activity of Fluoroquinolone Derivatives Containing a Chiral 3-(Alkoxyimino)-2-(aminomethyl)azetidine Moiety. ChemMedChem 2012, 7, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Guo, Q.; Wang, Y.C.; Liu, B.Q.; Liu, M.L.; Sun, L.Y.; Guo, H.Y. Synthesis and antibacterial activity of 7-(3-amino-4-alkoxyimino-1-piperidyl)-quinolones. Acta Pharm. Sin. 2008, 43, 819–827. [Google Scholar]
- Jia, X.D.; Wang, S.; Wang, M.H.; Liu, M.L.; Xia, G.M.; Liu, X.J.; Chai, Y.; He, H.W. Synthesis and in vitro antitumor activity of novel naphthyridinone derivatives. Chin. Chem. Lett. 2016. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 23f–q are available from the authors.
| Compound | Z | % Inhibition a | IC50 (μM) | |||||
|---|---|---|---|---|---|---|---|---|
| MCF-7 | HepG2 | HT-29 | A549 | PANC-1 | SKOV-3 | |||
| 23a |  | 87.29 | 12.790 | 7.060 | 8.893 | 4.993 | 14.132 | 5.766 |
| 23b |  | 83.61 | 27.457 | 6.852 | 10.820 | 6.555 | 13.672 | 5.978 |
| 23c |  | 81.46 | 17.946 | 7.882 | 9.524 | 3.103 | 9.410 | 6.669 |
| 23d |  | 84.98 | 15.052 | 6.316 | 10.388 | 6.250 | 9.512 | 3.721 |
| 23e |  | 46.83 | 65.054 | 36.964 | 19.642 | 26.031 | 33.314 | 29.685 |
| Sunitinib | 27.79 | 65.606 | 41.805 | 31.774 | 29.257 | 54.916 | 31.985 | |
| Compound | Z | IC50(μM) | ||
|---|---|---|---|---|
| HepG2 | A549 | SKOV-3 | ||
| 23f |  | 7.144 | 6.982 | 5.023 |
| 23g |  | 7.803 | 6.137 | 7.507 |
| 23h |  | 11.543 | 13.506 | 15.126 |
| 23i |  | 7.657 | 6.254 | 6.470 |
| 23j |  | 14.916 | 13.443 | 14.916 |
| 23k |  | 12.229 | 10.273 | 10.998 |
| 23l |  | 60.617 | 59.319 | 40.087 |
| 23m |  | 99.667 | 119.493 | 25.090 |
| 23n |  | 22.054 | 36.509 | 17.296 |
| 23o |  | 6.828 | 7.731 | 7.747 |
| 23p |  | 3.012 | 2.357 | 2.659 |
| 23q |  | 5.878 | 6.681 | 4.075 |
| Sunitinib | 33.999 | 31.594 | 49.036 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shen, W.; Li, X.; Chai, Y.; Li, S.; Lv, K.; Guo, H.; Liu, M. Synthesis and Antitumor Activity of 5-Bromo-7-azaindolin-2-one Derivatives Containing a 2,4-Dimethyl-1H-pyrrole-3-carboxamide Moiety. Molecules 2016, 21, 1674. https://doi.org/10.3390/molecules21121674
Zhang J, Shen W, Li X, Chai Y, Li S, Lv K, Guo H, Liu M. Synthesis and Antitumor Activity of 5-Bromo-7-azaindolin-2-one Derivatives Containing a 2,4-Dimethyl-1H-pyrrole-3-carboxamide Moiety. Molecules. 2016; 21(12):1674. https://doi.org/10.3390/molecules21121674
Chicago/Turabian StyleZhang, Jun, Weiyi Shen, Xiaoning Li, Yun Chai, Senjun Li, Kai Lv, Huiyuan Guo, and Mingliang Liu. 2016. "Synthesis and Antitumor Activity of 5-Bromo-7-azaindolin-2-one Derivatives Containing a 2,4-Dimethyl-1H-pyrrole-3-carboxamide Moiety" Molecules 21, no. 12: 1674. https://doi.org/10.3390/molecules21121674