Bioassay-Guided Isolation of Cytotoxic Isocryptoporic Acids from Cryptoporus volvatus
Abstract
:1. Introduction
2. Results and Discussion
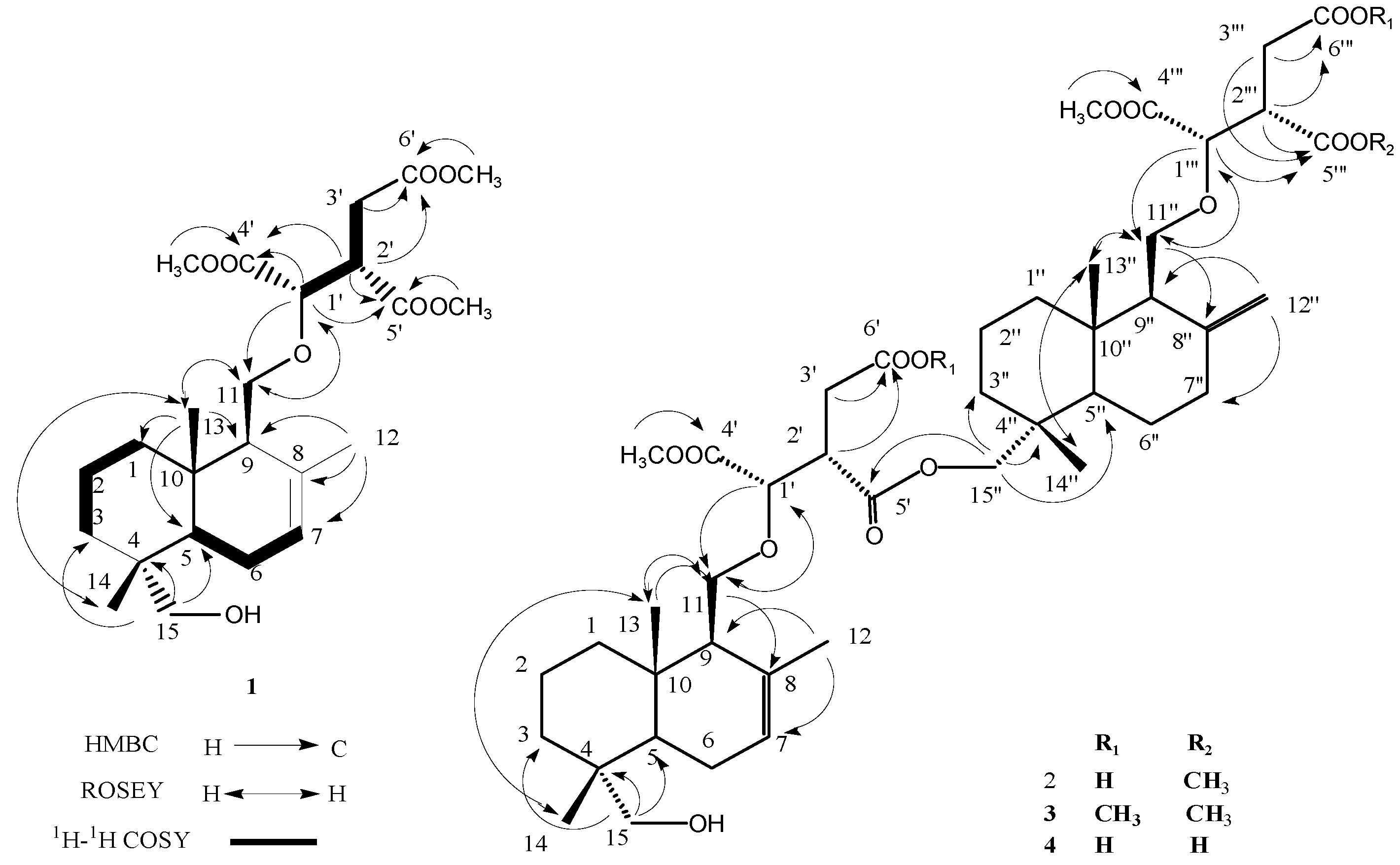
2.1. Identification of New Compounds
2.2. Cytotoxic Activities
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Assays of Cytotoxic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, J.D. Flora FungorumSinicorum; Science Press: Beijing, China, 1998; pp. 88–89. [Google Scholar]
- Hirotani, M.; Furuya, T.; Shiro, M. Cryptoporic acid-H and acid-I, drimane-sesquiterpenes from ganoderma-neo-japonicum and Cryptoporus volvatus. Phytochemistry 1991, 30, 1555–1559. [Google Scholar] [CrossRef]
- Asakaw, Y.; Hashimoto, T.; Mizuno, Y.; Toria, M.; Fukazawa, Y. Cryptoporicacids AG, drimane-type sesquiterpenoid ethers of isocitric acid from the fungus Cryptoporus volvatus. Phytochemistry 1992, 31, 579–592. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, G.Z.; Gao, L.; Cao, L.; Lv, N.; Shen, L.G.; Si, J.Y. Two new cryptoporic acid derivatives from the fruiting bodies of Cryptoporus volvatus. Phytochem. Lett. 2015, 14, 64–66. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, G.Z.; Lv, N.; Gao, L.; Cao, L.; Shen, L.G.; Si, J.Y. Chemical constituents from the fruiting bodies of Cryptoporusvolvatus. Arch. Pharm. Res. 2016, 39, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, F.; Ding, R.; Bao, L.; Gao, H.; Lu, J.C.; Yao, X.S.; Zhang, X.Q.; Liu, H.W. Four New Cryptoporic Acid Derivatives from the Fruiting Bodies of Cryptoporus sinensis, and Their Inhibitory Effects on Nitric Oxide Production. Chem. Biodivers. 2011, 8, 1529–1538. [Google Scholar] [CrossRef]
- Xu, X.B.; Gu, Z.X.; Liu, S.; Gao, N.; He, X.Z.; Xin, X. Purification and characterization of a glucan from Bacillus Calmette Guerin and the antitumor activity of its sulfated derivative. Carbohydr. Polym. 2015, 128, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Tori, M.; Hamada, N.; Sono, M.; Sono, Y.; Ishikawa, M.; Nakashima, K.; Hashimoto, T.; Asakawa, Y. Synthesis of cryptoporic acid A methyl ester. Tetrahedron Lett. 2000, 41, 3099–3102. [Google Scholar] [CrossRef]
- Huang, K.Y.; Kao, S.H.; Wang, W.L.; Chen, C.Y.; Hsiao, T.H.; Salunke, S.B.; Chen, J.W.; Su, C.K.; Yang, S.C.; Hong, T.M.; et al. Small Molecule T315 Promotes Casitas B-Lineage Lymphoma-Dependent Degradation of Epidermal Growth Factor Recptor via Y1045 Autophosphorylation. Am. J. Respir. Crit. Care Med. 2016, 193, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors becuase of the limited amount.
| H | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 1.03 (m), 1.93 (m) | 1.03 (m), 1.88 (m) | 1.03 (m), 1.84 (m) | 1.03 (m), 1.88 (m) |
| 2 | 1.51 (m), 1.62 (m) | 1.50 (m), 1.63 (m) | 1.48 (m), 1.63 (m) | 1.50 (m), 1.63 (m) |
| 3 | 1.33 (m), 1.45 (m) | 1.34 (m), 1.42 (m) | 1.33 (m), 1.42 (m) | 1.32 (m), 1.42 (m) |
| 5 | 1.48 (m) | 1.52 (m) | 1.52 (m) | 1.53 (m) |
| 6 | 1.82 (m) | 1.88 (m) | 1.86 (m) | 1.86 (m) |
| 7 | 5.43 (brs) | 5.42 (brs) | 5.41 (brs) | 5.44 (brs) |
| 9 | 1.95 (m) | 1.94 (m) | 1.93 (m) | 1.94 (m) |
| 11 | 3.43 (dd, 9.8, 3.2) | 3.46 (m) | 3.43 (m) | 3.48 (m) |
| 3.80 (dd, 9.8, 6.0) | 3.84 (m) | 3.86 (m) | 3.82 (m) | |
| 12 | 1.87 (brs) | 1.73 (brs) | 1.70 (brs) | 1.75 (brs) |
| 13 | 0.83 (s) | 0.84 (s) | 0.83 (s) | 0.84 (s) |
| 14 | 0.84 (s) | 0.84 (s) | 0.84 (s) | 0.85 (s) |
| 15 | 3.11 (d, 10.8) | 3.12 (d, 11.2) | 3.11 (d, 11.2) | 3.12 (d, 11.2) |
| 3.35 (d, 10.8) | 3.35 (d, 11.2) | 3.35 (d, 11.2) | 3.37 (d, 11.2) | |
| 1′ | 4.09 (d, 4.2) | 4.10 (d, 4.8) | 4.08 (d, 4.8) | 4.11 (d, 4.8) |
| 2′ | 3.45 (m) | 3.41 (m) | 3.43 (m) | 3.43 (m) |
| 3′ | 2.53 (dd, 16.8, 4.4) | 2.64 (m) | 2.55 (m) | 2.64 (m) |
| 2.80 (dd, 16.8, 9.2) | 2.82 (m) | 2.78 (m) | 2.80 (m) | |
| 1′′ | 1.13 (m), 1.66 (m) | 1.15 (m), 1.68 (m) | 1.12 (m), 1.65 (m) | |
| 2′′ | 1.52 (m), 1.63 (m) | 1.50 (m), 1.63 (m) | 1.50 (m), 1.66 (m) | |
| 3′′ | 1.21 (m), 1.58 (m) | 1.23 (m), 1.58 (m) | 1.20 (m), 1.58 (m) | |
| 5′′ | 1.34 (m) | 1.31 (m) | 1.35 (m) | |
| 6′′ | 1.35 (m), 1.57 (m) | 1.33 (m), 1.55 (m) | 1.34 (m), 1.55 (m) | |
| 7′′ | 2.00 (m), 2.38 (m) | 2.01 (m), 2.36 (m) | 2.01 (m), 2.38 (m) | |
| 9′′ | 1.99 (m) | 1.96 (m) | 2.03 (m) | |
| 11′′ | 3.53 (m) | 3.51 (m) | 3.55 (m) | |
| 3.92 (m) | 3.89 (m) | 3.94 (m) | ||
| 12′′ | 4.78 (brs), 4.84 (brs) | 4.75 (brs), 4.84 (brs) | 4.78 (brs), 4.86 (brs) | |
| 13′′ | 0.74 (s) | 0.75 (s) | 0.75 (s) | |
| 14′′ | 0.75 (s) | 0.74 (s) | 0.76 (s) | |
| 15′′ | 3.62 (d, 11.2) | 3.65 (d, 11.2) | 3.45 (d, 11.2) | |
| 4.06 (d, 11.2) | 3.86 (d, 11.2) | 3.96 (d, 11.2) | ||
| 1′′′ | 4.14 (d, 4.8) | 4.10 (d, 4.8) | 4.18 (d, 4.8) | |
| 2′′′ | 3.40 (m) | 3.42 (m) | 3.38 (m) | |
| 3′′′ | 2.72 (m) | 2.53 (m) | 2.60 (m) | |
| 2.75 (m) | 2.78 (m) | 2.68 (m) | ||
| OMe | 3.65 (s) | 3.68 (s) | 3.67 (s) | 3.75 (s) |
| 3.65 (s) | 3.76 (s) | 3.67 (s) | 3.76 (s) | |
| 3.68 (s) | 3.76 (s) | 3.68 (s) | ||
| 3.74 (s) | ||||
| 3.75 (s) |
| C | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 39.1 | 38.8 | 39.0 | 38.7 |
| 2 | 18.1 | 18.5 | 18.3 | 18.5 |
| 3 | 35.5 | 35.4 | 35.4 | 35.3 |
| 4 | 35.5 | 37.4 | 37.4 | 37.8 |
| 5 | 44.5 | 44.6 | 44.6 | 44.7 |
| 6 | 23.4 | 23.4 | 23.4 | 23.4 |
| 7 | 122.5 | 122.5 | 122.5 | 123.0 |
| 8 | 133.4 | 133.2 | 133.4 | 132.9 |
| 9 | 54.8 | 54.9 | 54.7 | 55.7 |
| 10 | 37.5 | 38.5 | 38.5 | 38.5 |
| 11 | 69.5 | 68.9 | 69.7 | 68.4 |
| 12 | 21.7 | 21.8 | 21.8 | 21.8 |
| 13 | 15.2 | 15.7 | 15.7 | 15.7 |
| 14 | 17.7 | 17.6 | 17.5 | 17.6 |
| 15 | 72.0 | 71.8 | 71.9 | 71.5 |
| 1′ | 78.7 | 78.4 | 78.5 | 77.7 |
| 2′ | 43.3 | 43.2 | 43.2 | 43.4 |
| 3′ | 31.9 | 32.0 | 32.1 | 32.3 |
| 4′ | 171.1 | 171.1 | 171.2 | 172.6 |
| 5′ | 170.8 | 170.7 | 170.8 | 170.8 |
| 6′ | 172.2 | 175.0 | 172.2 | 174.9 |
| 1′′ | 38.5 | 38.5 | 38.5 | |
| 2′′ | 18.1 | 18.0 | 18.4 | |
| 3′′ | 35.3 | 35.6 | 35.3 | |
| 4′′ | 37.6 | 37.1 | 37.8 | |
| 5′′ | 48.9 | 48.9 | 48.3 | |
| 6′′ | 23.6 | 23.6 | 23.6 | |
| 7′′ | 36.8 | 36.7 | 36.8 | |
| 8′′ | 146.3 | 146.3 | 146.5 | |
| 9′′ | 55.1 | 54.8 | 55.3 | |
| 10′′ | 38.9 | 39.0 | 38.7 | |
| 11′′ | 68.2 | 68.2 | 68.4 | |
| 12′′ | 108.2 | 108.2 | 108.2 | |
| 13′′ | 15.6 | 15.6 | 15.6 | |
| 14′′ | 17.7 | 17.6 | 17.7 | |
| 15′′ | 73.6 | 73.8 | 73.2 | |
| 1′′′ | 78.8 | 78.9 | 78.8 | |
| 2′′′ | 44.6 | 44.4 | 44.7 | |
| 3′′′ | 31.7 | 31.7 | 32.0 | |
| 4′′′ | 170.9 | 170.8 | 171.1 | |
| 5′′′ | 171.1 | 170.7 | 175.0 | |
| 6′′′ | 175.2 | 172.1 | 176.0 | |
| OMe | 52.0 | 52.1 | 51.9 | 52.2 |
| 52.0 | 52.2 | 51.9 | 52.2 | |
| 52.2 | 52.2 | 52.0 | ||
| 52.0 | ||||
| 52.0 |
| Samples | IC50 (μg/mL) | ||||
|---|---|---|---|---|---|
| HL-60 | A-549 | SMMC-7721 | MCF-7 | SW480 | |
| The EtOAc extract | >200 | >300 | >300 | >300 | >300 |
| The MeOH extract | >200 | >300 | >300 | >300 | >300 |
| The 60% EtOH-H2O extract | >300 | >500 | >400 | >400 | >400 |
| Fraction 1 | >300 | >400 | >400 | >400 | >400 |
| Fraction 2 | >200 | >300 | >200 | >200 | >200 |
| Fraction 3 | 80.81 ± 1.90 | >100 | 95.19 ± 2.80 | >100 | 87.82 ± 0.50 |
| Fraction 4 | 70.22 ± 1.70 | >100 | 88.97 ± 1.54 | 92.14 ± 1.90 | 80.13 ± 3.30 |
| Fraction 5 | 67.35 ± 1.30 | 94.28 ± 1.35 | 85.53 ± 0.98 | 90.62 ± 1.50 | 85.67 ± 1.30 |
| Fraction 6 | 80.87 ± 2.90 | >100 | >100 | >100 | 97.14 ± 1.80 |
| Fraction 7 | 98.26 ± 1.90 | >100 | >100 | >100 | >100 |
| Fraction 8 | >100 | >300 | >200 | >200 | >200 |
| Compounds | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HL-60 | A-549 | SMMC-7721 | MCF-7 | SW480 | |
| 4 | 20.92 ± 1.20 | 26.91 ± 1.80 | 21.19 ± 2.10 | 26.62 ± 1.60 | 17.82 ± 0.70 |
| Cis-platin | 1.68 ± 0.22 | 25.22 ± 1.15 | 14.85 ± 0.82 | 11.77 ± 2.28 | 4.28 ± 1.51 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.-Y.; Yu, X.-H.; Lu, B.; Hua, Y. Bioassay-Guided Isolation of Cytotoxic Isocryptoporic Acids from Cryptoporus volvatus. Molecules 2016, 21, 1692. https://doi.org/10.3390/molecules21121692
Zhou L-Y, Yu X-H, Lu B, Hua Y. Bioassay-Guided Isolation of Cytotoxic Isocryptoporic Acids from Cryptoporus volvatus. Molecules. 2016; 21(12):1692. https://doi.org/10.3390/molecules21121692
Chicago/Turabian StyleZhou, Ling-Yun, Xiao-Hong Yu, Bin Lu, and Yan Hua. 2016. "Bioassay-Guided Isolation of Cytotoxic Isocryptoporic Acids from Cryptoporus volvatus" Molecules 21, no. 12: 1692. https://doi.org/10.3390/molecules21121692