Polyphenols Isolated from Xanthoceras sorbifolia Husks and Their Anti-Tumor and Radical-Scavenging Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Chemotaxonomic Significance
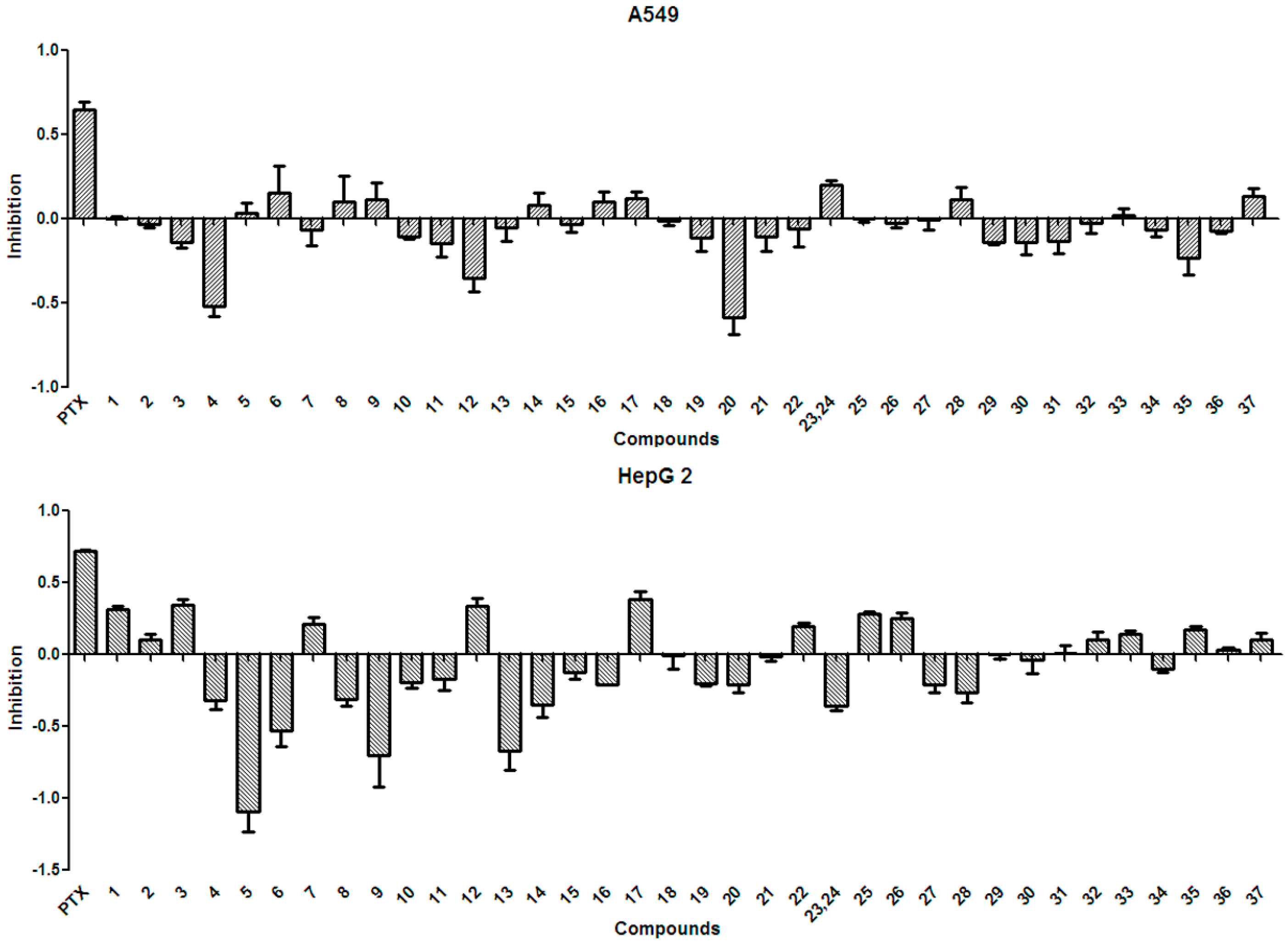
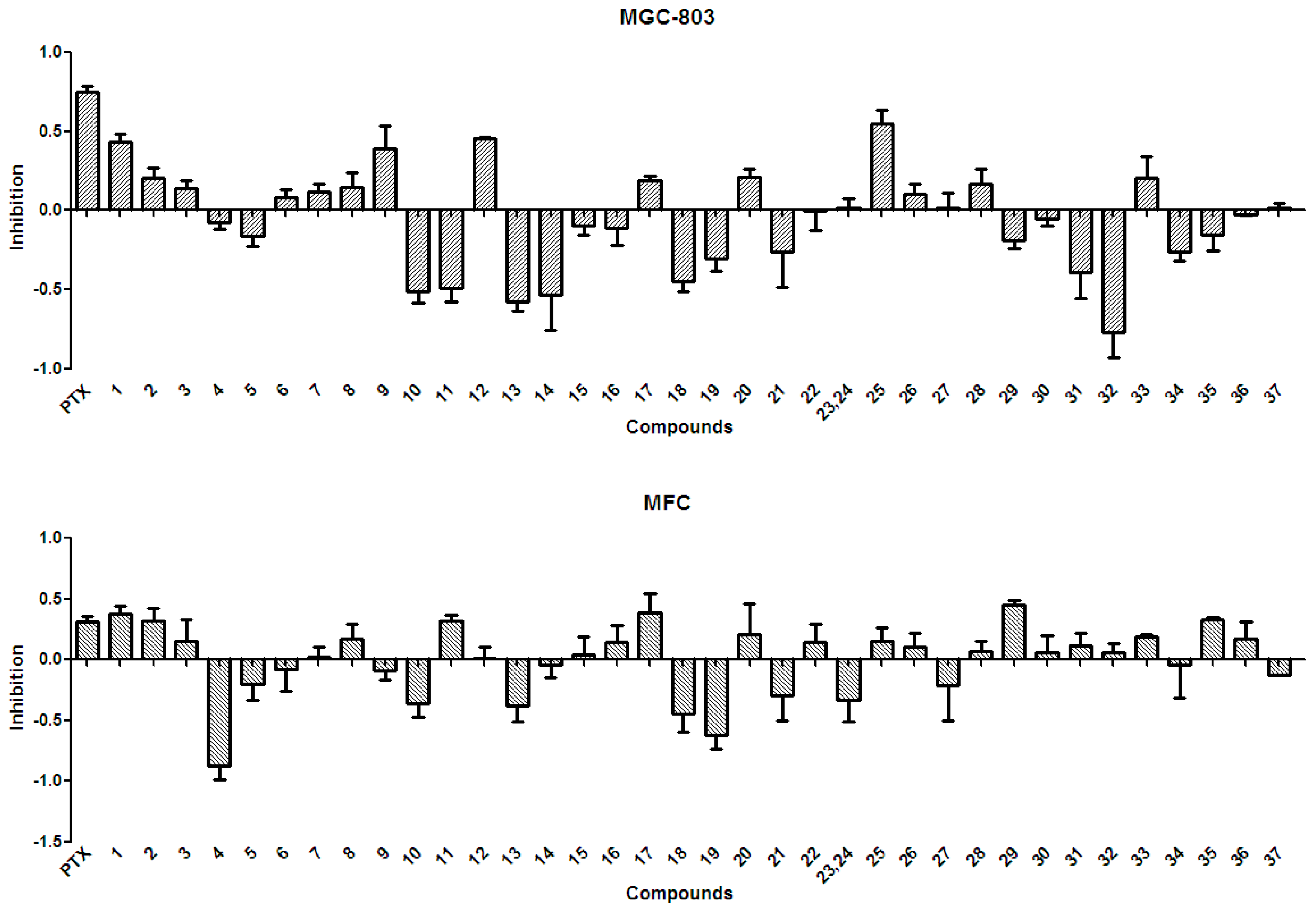
2.3. Anti-Tumor Effects of X. sorbifolia Polyphenols
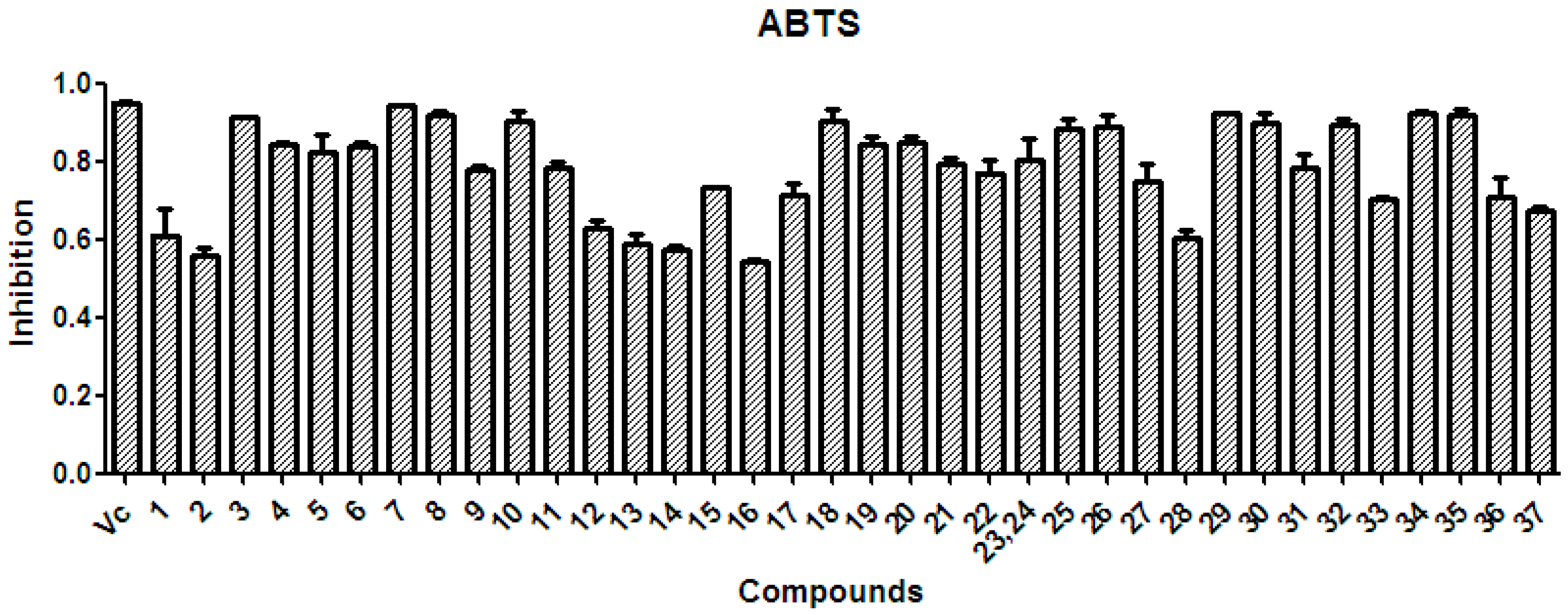
2.4. Radical-Scavenging Activity of Extracts and Polyphenols
2.4.1. Radical-Scavenging Activity
2.4.2. Discussion on the SAR of Polyphenols
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material and Reagents
3.3. Extraction and Isolation
3.4. Acidic Hydrolysis of the New Compound and Sugar Analysis
3.5. Biological Activity
3.5.1. Anti-Tumor Assay
3.5.2. ABTS Radical-Scavenging Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.Y.; Xiang, Z.; Cui, H.; Xiao, H.; Kang, T.G.; Dou, D.Q.; Kuang, H.X. Two new oleanane-type saponins from the husks of Xanthoceras sorbifolia Bunge. Nat. Prod. Res. 2013, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Wang, X.B.; Wei, X.C.; Wang, M.M.; Chen, L.X.; Cao, S.J.; Kang, N.; Qiu, F. Triterpenoid saponins from Xanthoceras sorbifolia Bunge. and their inhibitory activity on human cancer cell lines. Bioorg. Med. Chem. Lett. 2012, 22, 5232–5238. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, S.C.; Dou, D.Q. Determination of the content of total saponins in the husks of Xanthoceras sorbifolia Bunge. J. Liaoning Univ. TCM 2009, 11, 165–166. [Google Scholar]
- Wan, G.S.; Ren, Y.H.; Gao, H.Y.; Bai, S.; Xi, R.G.; Wang, X.B. Isolation and identification of chemical constituents from the husks of Xanthoceras sorbifolium Bunge. J. Shenyang Pharm. Univ. 2015, 32, 18–21. [Google Scholar]
- Li, Z.L.; Li, X.; Li, L.H.; Li, N.; Yu, M.; Meng, D.L. Two new triterpenes from the husks of Xanthoceras sorbifolia. Planta Med. 2005, 71, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Li, X.; Li, L.; Li, W. Studies on the chemical constituents of the husks of Xanthoceras sorbifolia Bunge. J. Shenyang Pharm. Univ. 2005, 22, 271–272. [Google Scholar]
- Ji, X.F.; Chi, T.Y.; Xu, Q.; He, X.L.; Zhou, X.Y.; Zhang, R.; Zou, L.B. Xanthoceraside ameliorates mitochondrial dysfunction contributing to the improvement of learning and memory impairment in mice with intracerebroventricular injection of a beta 1–42. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zou, L.B.; Liu, P.; Xu, Q.; Zhang, Y.F.; Yu, Y.; Zou, L.; Chi, T.Y.; Ji, X.F. Xanthoceraside induces apoptosis in melanoma cells through the activation of caspases and the suppression of the IGF-1R/Raf/MEK/ERK signaling pathway. J. Med. Food 2014, 17, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Gen, J.; Zhou, Q.C. Inhibitory effect of flavonoids consituents from Xanthoceras Sorbifolia nutshell on tyrosinase. J. Chin. Cereals Oils Assoc. 2013, 28, 96–100. [Google Scholar]
- Xu, J.K.; Zhang, W.; Li, Y.J.; Ji, X.F.; Chi, T.Y.; Zou, L.B. Effects of xanthoceraside on focal cerebral ischemia reperfusion injury in rats and the preliminary mechanism study. J. Shenyang Pharm. Univ. 2014, 31, 793–798. [Google Scholar]
- Wu, W.J. Study on the Separation Process and Antioxidant Activity of the Total Sorbifolia Sheel Saponin; Beijing Forestry University: Beijing, China, 2010. [Google Scholar]
- Gen, J.; Zhang, H.M.; Zhou, Q.C. Inhibitory effect of Xanthoceras sorbifolia nutshell saponin on pancreatic lipase. Mod. Food Sci. Technol. 2014, 30, 89–92. [Google Scholar]
- Katalinic, M.; Rusak, G.; Barovic, J.D.; Sinko, G.; Jelic, D.; Antolovic, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Keawsa-ard, S.; Natakankitkul, S.; Liawruangrath, S.; Teerawutgulrag, A.; Trisuwan, K.; Charoenying, P.; Pyne, S.G.; Liawruangrath, B. Anticancer and antibacterial activities of the isolated compounds from Solanum spirale Roxb. leaves. Chiang Mai J. Sci. 2012, 39, 445–454. [Google Scholar]
- Lei, J.C.; Yang, C.X.; Yang, Y.; Zhang, W.; Yu, J.Q. Antioxidant and antitumour activities of extracts from Patrinia villosa and its active constituents. J. Funct. Foods 2015, 16, 289–294. [Google Scholar] [CrossRef]
- Zhao, S.M.; Chou, G.X.; Wang, Z.T. Chemical constituents from roots and rhizomes of Physochlaina infundibularis. Chin. Tradit. Herb. Drugs 2013, 44, 938–941. [Google Scholar]
- Zhang, W.K.; WANG, S.B.; Fu, C.Y.; Li, P.; Xu, J.K. Flavonoids from Humulus lupulus. China J. Chin. Meter. Med. 2013, 38, 1539–1542. [Google Scholar]
- Lotti, C.; Piccinelli, A.L.; Arevalo, C.; Ruiz, I.; De Castro, G.M.M.; De Sa, L.F.R.; Tessis, A.C.; Ferreira-Pereira, A.; Rastrelli, L. Constituents of Honduran propolis with inhibitory effects on Saccharomyces cerevisiae multidrug resistance protein pdr5p. J. Agric. Food. Chem. 2012, 60, 10540–10545. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Zhao, Q.C.; Yang, L.; Shang, Z.P.; Du, Z.Q.; Yan, M. Chemical constituents in roots of Paeonia lactiflora. Chin. Tradit. Herb. Drugs 2010, 41, 1245–1248. [Google Scholar]
- Kayano, S.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatani, N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. J. Agric. Food Chem. 2002, 50, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, J.; Wang, N.L.; Yao, X.S. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Bellakhdar, J.; Passannanti, S.; Paternostro, M.P.; Piozzi, F. Constituents of Origanum-Compactum. Planta Med. 1988, 94. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Saito, T.; Ishikawa, H.; Date, H.; Kataoka, S.; Tamura, Y.; Mizutani, K. Antiperoxidative components in Thymus vulgaris. Planta Med. 1996, 62, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Sakushima, A.; Coskun, M.; Hisada, S.; Nishibe, S. Flavonoids from Rhamnus-Pallasii. Phytochemistry 1983, 22, 1677–1678. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Dai, Y.; Gao, H.; Ye, W.C.; Yao, X.S. Study on chemical constituents of Sarcandra glaber. Chin. Tradit. Herb. Drugs 2010, 41, 29–32. [Google Scholar]
- Zhang, S.X.; Tani, T.; Yamaji, S.; Ma, C.M.; Wang, M.C.; Cai, S.Q.; Zhao, Y.Y. Glycosyl flavonoids from the roots and rhizomes of Asarum longerhizomatosum. J. Asian Nat. Prod. Res. 2003, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase principles and constituents of the petals of Crocus sativus. J. Nat. Prod. 2004, 67, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Lee, S.S. Dibenzocycloheptanoids from the leaves of Cinnamomum subavenium. J. Nat. Prod. 2012, 75, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Cui, C.B. One new and nine known flavonoids from Choerospondias axillaries and their in vitro antitumor, anti-hypoxia and antibacterial activities. Molecules 2014, 19, 21363–21377. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.F.; Zhou, B.; Huang, J.M.; Gao, X.M.; Shu, L.D.; Yang, G.Y.; Che, C.T. Antiviral phenolic compounds from Arundina gramnifolia. J. Nat. Prod. 2013, 76, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.H.; Yang, S.J.; Liang, N.; Hu, D.Y.; Jin, L.H.; Xue, W.; Yang, S. Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules 2013, 18, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Wang, F. A new flavanone glucoside from the flowers of Carthamus tinctorius and assignment of absolute configuration. Chem. Nat. Compd. 2014, 50, 427–429. [Google Scholar] [CrossRef]
- Gao, H.; Nishida, J.; Saito, S.; Kawabata, J. Inhibitory effects of 5,6,7-trihydroxyflavones on tyrosinase. Molecules 2007, 12, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Huang, M.Y.; Wang, F.; Liu, Q.Y.; Zhang, L.X.; Zhang, Y.H. Chemical constituents of Ajania nematoloba. Chem. Nat. Compd. 2015, 51, 143–145. [Google Scholar] [CrossRef]
- Funari, C.S.; Gullo, F.P.; Napolitano, A.; Carneiro, R.L.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M.; Piacente, S.; Pizza, C.; Silva, D.H.S. Chemical and antifungal investigations of six Lippia species (Verbenaceae) from Brazil. Food Chem. 2012, 135, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kurita, M.; Shinozaki, T.; Ukiya, M.; Yasukawa, K.; Shimizu, N.; Tokuda, H.; Masters, E.T.; Akihisa, M.; Akihisa, T. Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities. Phytochemistry 2014, 108, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.Y.; Zhang, C.H. Studies on the chemical constituents of Xanthoceras sorbifolia. J. Chin. Med. Mater. 2009, 32, 702–704. [Google Scholar]
- Jung, M.; Choi, J.; Chae, H.S.; Cho, J.Y.; Kim, Y.D.; Htwe, K.M.; Lee, W.S.; Chin, Y.W.; Kim, J.; Yoon, K.D. Flavonoids from Symplocos racemosa. Molecules 2015, 20, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, L.; Saturnino, P.; Schettino, O.; Dini, A. Studies on the constituents of Chenopodium Pallidicaule (Canihua) seeds-isolation and characterization of 2 new flavonol glycosides. J. Agric. Food Chem. 1995, 43, 2020–2024. [Google Scholar] [CrossRef]
- Tai, Z.G.; Zhang, F.M.; Cai, L.; Shi, J.; Cao, Q.E.; Ding, Z.T. Flavonol glycosides of Pseudodrynaria coronans and their antioxidant activity. Chem. Nat. Compd. 2012, 48, 221–224. [Google Scholar] [CrossRef]
- Aderogba, M.A.; Ndhlala, A.R.; Rengasamy, K.R.R.; Van Staden, J. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules 2013, 18, 12633–12644. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Lou, G.D.; Ma, Z.J.; Liu, X.M. Chemical constituents with antioxidant activities from litchi (Litchi chinensis Sonn.) seeds. Food Chem. 2011, 126, 1081–1087. [Google Scholar] [CrossRef]
- Lou, H.X.; Yamazaki, Y.; Sasaki, T.; Uchida, M.; Tanaka, H.; Oka, S. A-type proanthocyanidins from peanut skins. Phytochemistry 1999, 51, 297–308. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and related-compounds Structures of novel fermentation products, theogallinin, theaflavonin and desgalloyl theaflavonin from black tea, and Changes of tea leaf polyphenols during fermentation. Chem. Pharm. Bull. 1992, 40, 1383–1389. [Google Scholar] [CrossRef]
- Peng, X.; Yu, D.Y.; Feng, B.M.; Wang, Y.Q.; Shi, L.Y. A new acylated flavonoid glycoside from the flowers of Camellia nitidissima and its effect on the induction of apoptosis in human lymphoma U937 cells. J. Asian Nat. Prod. Res. 2012, 14, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Panyadee, A.; Sahakitpichan, P.; Ruchirawat, S.; Kanchanapoom, T. 5-methyl ether flavone glucosides from the leaves of Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 11, 215–219. [Google Scholar] [CrossRef]
- Liao, C.R.; Kuo, Y.H.; Ho, Y.L.; Wang, C.Y.; Yang, C.S.; Lin, C.W.; Chang, Y.S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 Cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Wu, D.; Jiang, Y.M.; Prasad, K.N.; Lin, S.; Jiang, G.X.; He, J.R.; Zhao, M.M.; Luo, W.; Yang, B. Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities. J. Funct. Foods 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Chang, R.J.; Wang, C.H.; Zeng, Q.; Guan, B.; Zhang, W.D.; Jin, H.Z. Chemical constituents of the stems of Celastrus rugosus. Arch. Pharm. Res. 2013, 36, 1291–1301. [Google Scholar]
- Ashour, M.L.; El-Readi, M.Z.; Tahrani, A.; Eid, S.Y.; Wink, M. A novel cytotoxic aryltetraline lactone from Bupleurum marginatum (Apiaceae). Phytochem. Lett. 2012, 5, 387–392. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Huang, G.J.; Wang, B.S.; Lin, W.C.; Huang, S.S.; Lee, C.Y.; Yen, M.T.; Huang, M.H. Antioxidant and anti-inflammatory properties of Longan (Dimocarpus longan Lour.) pericarp. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, Z.H.; Qin, P.Y.; Yao, Y.; Meng, X.J.; Zou, J.Q.; Zhu, K.; Ren, G.X. Chemical characterization of a procyanidin-rich extract from Sorghum Bran and Its effect on oxidative stress and tumor inhibition in vivo. J. Agric. Food Chem. 2011, 59, 8609–8615. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Yang, C.Y.; Li, F.; Du, B.W.; Chen, B.; Wang, F.; Wang, M.K. Isolation and characterization of new phenolic compounds with estrogen biosynthesis-inhibiting and antioxidation activities from Broussonetia papyrifera leaves. PLoS ONE 2014, 9, e94198. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Liu, Z.Q.; Zou, K.; Wang, J.Z.; Zhou, Y.Q.; Liu, X.Q. Preliminary experimental study on the anti-tumor effect of Tupistra chinensis Bak. in vitro. Lishizhen Med. Mater. Med. Res. 2009, 20, 2390–2392. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.



| No. | 13C-NMR | DEPT | 1H-NMR |
|---|---|---|---|
| 1 | 106.5 | C | |
| 2 | 162.1 | C | |
| 3 | 100.5 | CH | 6.15 (br s) |
| 4 | 161.5 | C | |
| 5 | 110.6 | CH | 6.18 (br s) |
| 6 | 141.9 | C | |
| 6-CH3 | 22.8 | CH3 | 2.37 (s) |
| 7 | 170.2 | C | |
| 1′ | 67.7 | CH2 | 4.49 (dd, 1.6, 10.8), 4.24 (dd, 6.4, 11.2) |
| 2′ | 68.1 | CH | 3.79 (m) |
| 3′ | 69.7 | CH | 3.63 (m) |
| 4′ | 69.3 | CH | 3.58 (m) |
| 5′ | 71.2 | CH | 3.47 (m) |
| 6′ | 63.8 | CH2 | 3.62 (m), 3.40 (o) |
| No. | 13C-NMR | DEPT | 1H-NMR |
|---|---|---|---|
| 1 | 112.7 | C | |
| 2 | 117.0 | CH | 7.39 (d, 2.8) |
| 3 | 155.4 | C | |
| 4 | 149.7 | C | |
| 5 | 118.2 | CH | 6.94 (d, 9.2) |
| 6 | 125.5 | CH | 7.25 (dd, 2.8, 8.8) |
| 7 | 168.9 | C | |
| –OCH3 | 52.6 | CH3 | 3.90 (s) |
| 1′ | 101.9 | CH | 4.68 (d, 7.6) |
| 2′ | 73.2 | CH | 3.20 (o) |
| 3′ | 75.5 | CH | 3.42 (o) |
| 4′ | 70.0 | CH | 3.08 (t, 8.8) |
| 5′ | 76.3 | CH | 3.24 (m) |
| 6′ | 66.6 | CH2 | 3.85 (d, 9.2), 3.39 (o) |
| 1′′ | 100.6 | CH | 4.54 (s) |
| 2′′ | 70.3 | CH | 3.59 (br s) |
| 3′′ | 70.7 | CH | 3.43 (o) |
| 4′′ | 72.0 | CH | 3.16 (o) |
| 5′′ | 68.3 | CH | 3.41 (o) |
| 6′′ | 17.8 | CH3 | 1.10 (d, 6.0) |
| Compounds | SC50 a Values (µg/mL) | Compound | SC50 a Values (µg/mL) |
|---|---|---|---|
| ABTS | ABTS | ||
| 1 | >50 | 20 | 3.45 ± 1.38 |
| 2 | 12.59 ± 1.33 | 21 | 37.95 ± 1.87 |
| 3 | 4.72 ± 1.13 | 22 | 10.19 ± 1.11 |
| 4 | 5.99 ± 1.09 | 23, 24 | 12.73 ± 1.02 |
| 5 | 20.61 ± 1.07 | 25 | 3.65 ± 1.02 |
| 6 | 13.48 ± 1.08 | 26 | 4.76 ± 1.10 |
| 7 | 4.32 ± 1.09 | 27 | 32.31 ± 4.87 |
| 8 | 6.82 ± 1.18 | 28 | 28.65 ± 1.07 |
| 9 | >50 | 29 | 5.69 ± 1.13 |
| 10 | 5.85 ± 1.04 | 30 | 5.25 ± 1.07 |
| 11 | 10.19 ± 1.11 | 31 | 8.48 ± 1.15 |
| 12 | 11.16 ± 1.05 | 32 | 5.23 ± 1.18 |
| 13 | 24.44 ± 1.16 | 33 | 13.86 ± 1.10 |
| 14 | 52.33 ± 1.15 | 34 | 7.99 ± 1.11 |
| 15 | >50 | 35 | 7.46 ± 1.09 |
| 16 | 42.43 ± 1.54 | 36 | 9.05 ± 1.07 |
| 17 | 18.15 ± 1.27 | 37 | 14.04 ± 1.09 |
| 18 | 4.45 ± 1.06 | Ascorbic acid b | 7.67 ± 1.09 |
| 19 | 4.54 ± 1.36 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-Y.; Ha, W.; Lin, Y.; Jiang, K.; Yang, J.-L.; Shi, Y.-P. Polyphenols Isolated from Xanthoceras sorbifolia Husks and Their Anti-Tumor and Radical-Scavenging Activities. Molecules 2016, 21, 1694. https://doi.org/10.3390/molecules21121694
Yang C-Y, Ha W, Lin Y, Jiang K, Yang J-L, Shi Y-P. Polyphenols Isolated from Xanthoceras sorbifolia Husks and Their Anti-Tumor and Radical-Scavenging Activities. Molecules. 2016; 21(12):1694. https://doi.org/10.3390/molecules21121694
Chicago/Turabian StyleYang, Chun-Yan, Wei Ha, Yong Lin, Kan Jiang, Jun-Li Yang, and Yan-Ping Shi. 2016. "Polyphenols Isolated from Xanthoceras sorbifolia Husks and Their Anti-Tumor and Radical-Scavenging Activities" Molecules 21, no. 12: 1694. https://doi.org/10.3390/molecules21121694






