Enhanced Stilbene Production and Excretion in Vitis vinifera cv Pinot Noir Hairy Root Cultures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Establishment of Hairy Root Lines (HRs)
2.2. Effects of Culture Medium and Sucrose Concentration on HR Growth
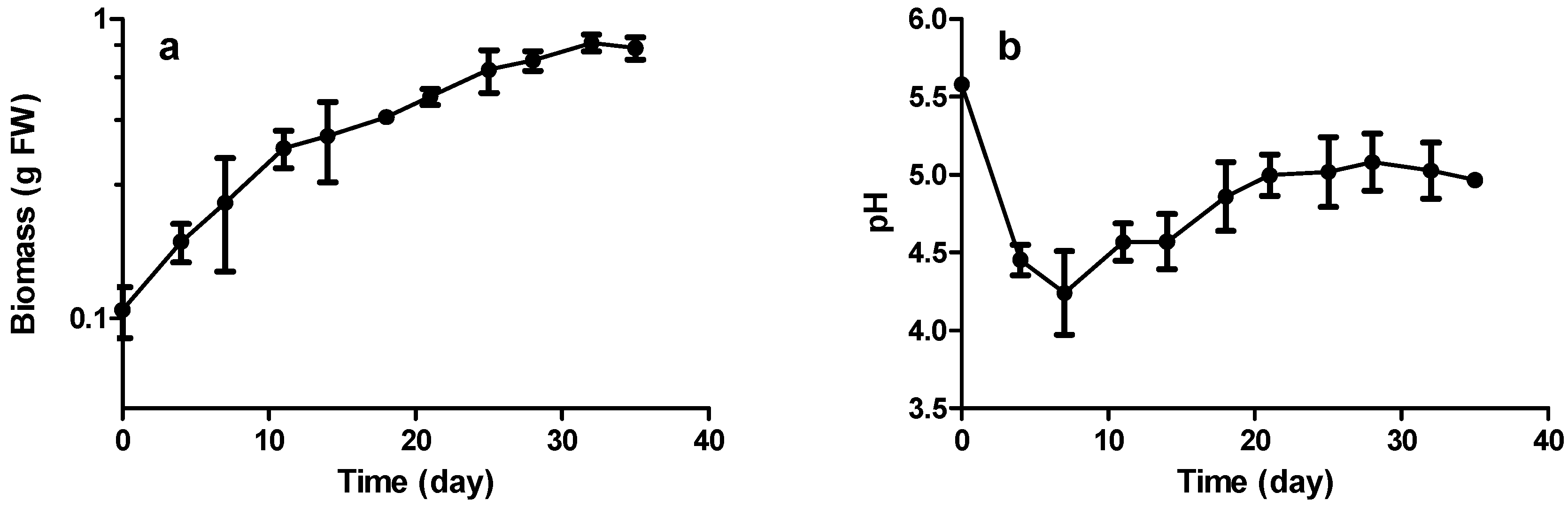
2.3. Growth and Stilbene Production Kinetics
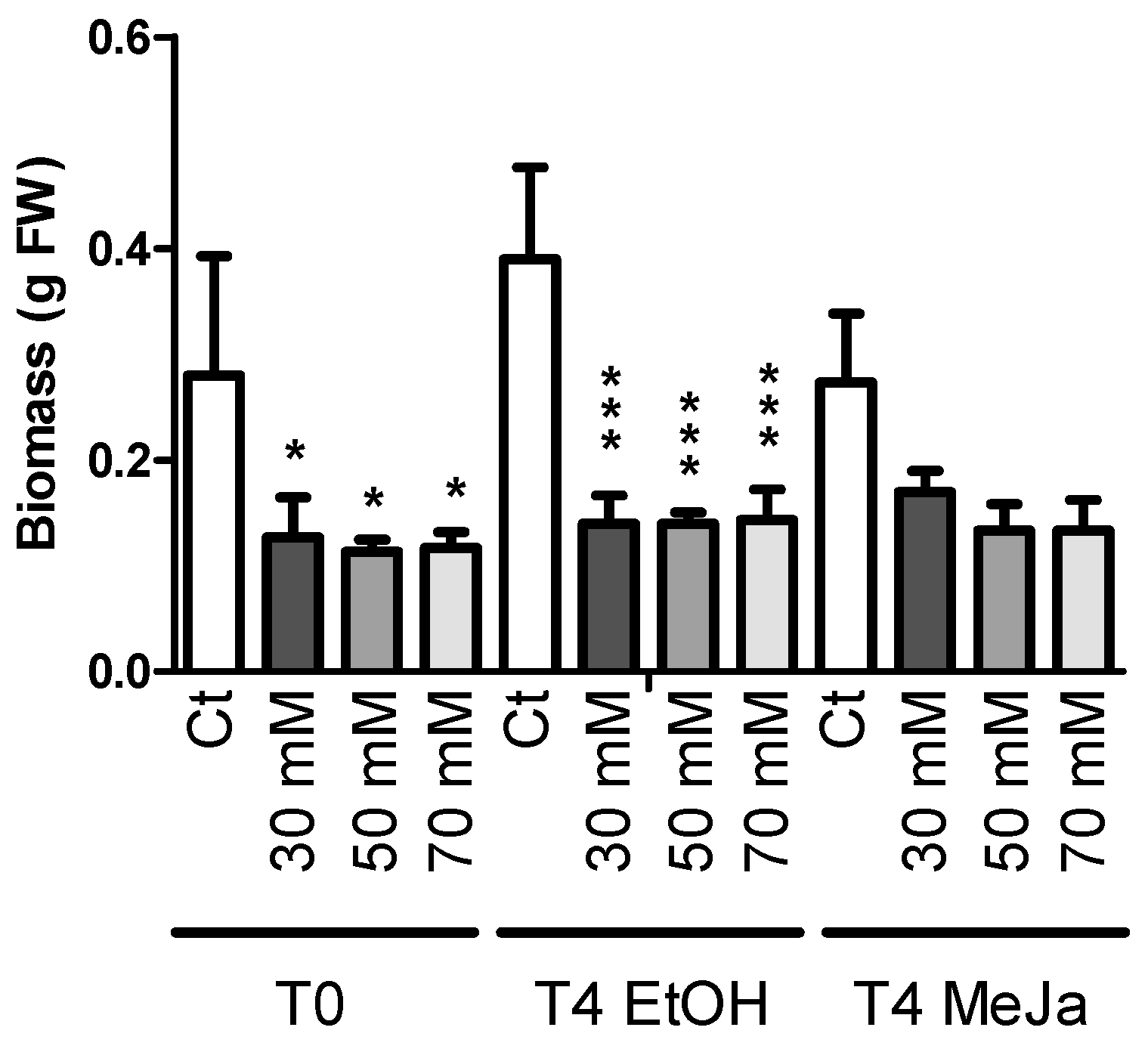
2.3.1. Biomass Production
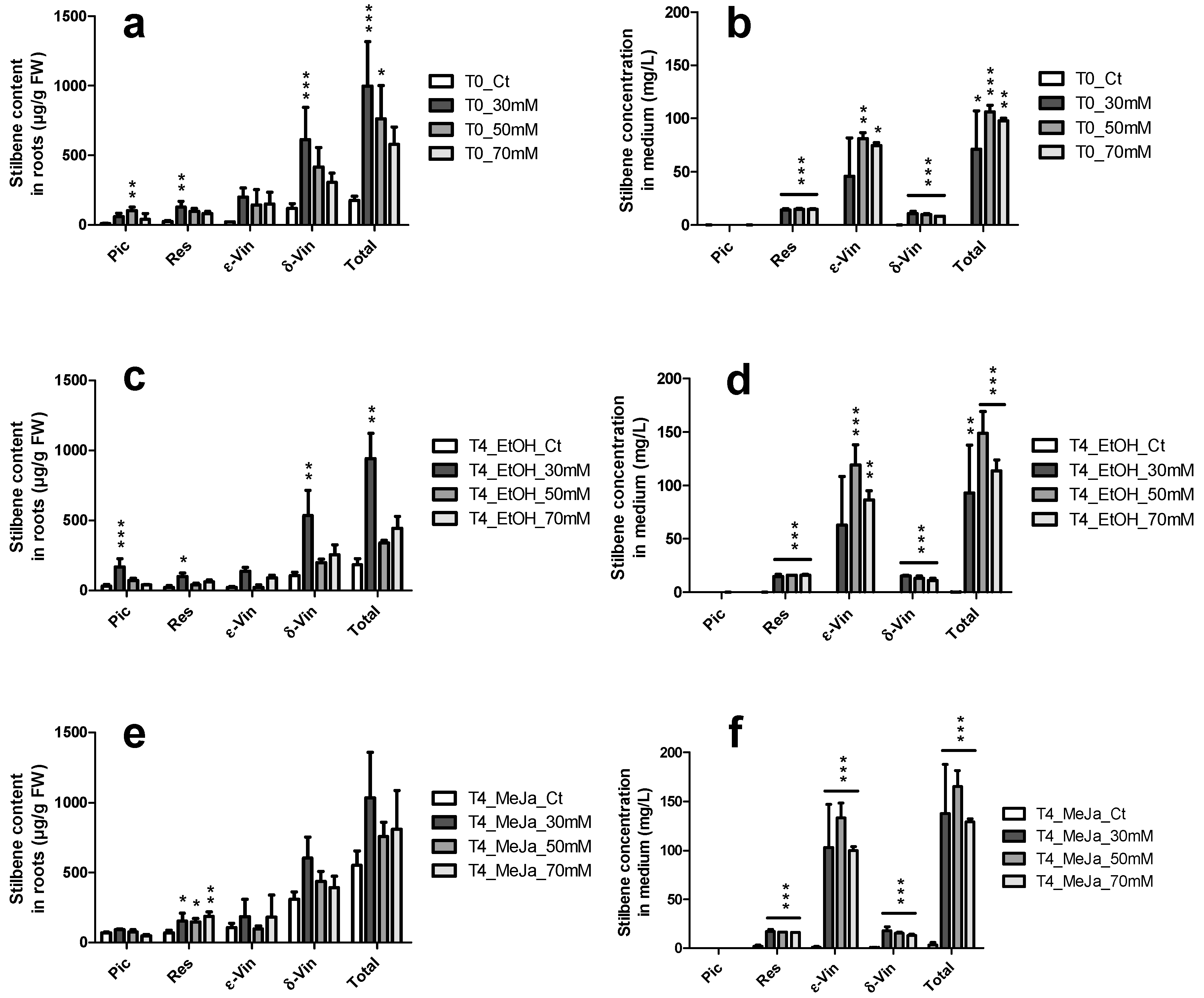
2.3.2. Constitutive Stilbene Production in Roots
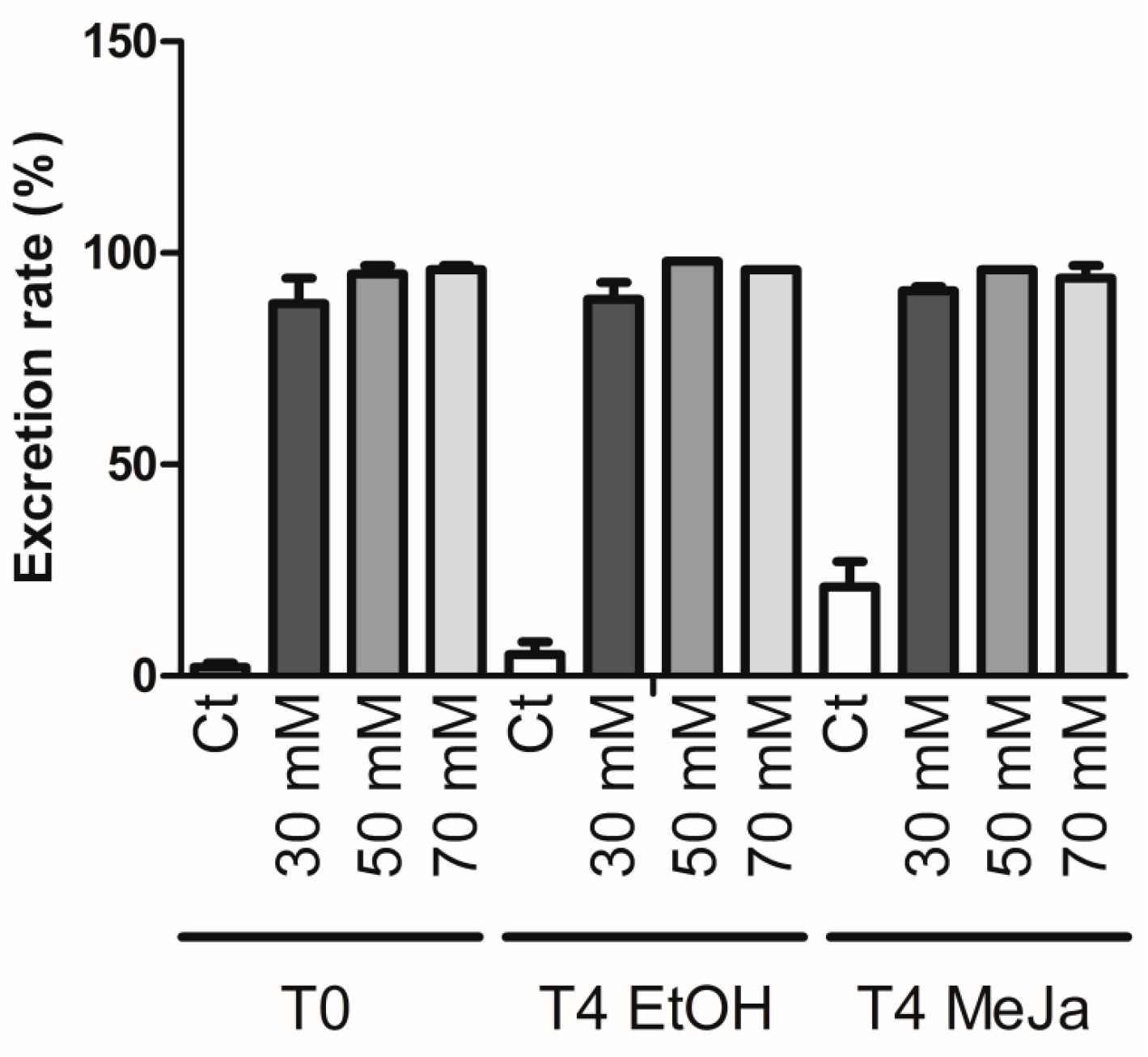
2.4. Induction of Stilbene Production in HRs in Response to Various Elicitors
2.4.1. Elicitation with Methyl Jasmonate
2.4.2. Elicitation Experiments with Cyclodextrins and/or Methyl Jasmonate
3. Materials and Methods
3.1. Plant and Bacterial Materials
3.2. Confirmation of the Genetic Transformation
3.3.Improvement of Growth Conditions
3.4. Growth Kinetics
3.5. Stilbene Extraction
3.6. UPLC Analysis
3.7. Induction Treatments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| B5 | Gamborg medium |
| CD | Cyclodextrin |
| HR | Hairy roots |
| JA | Jasmonate |
| MeJA | Methyl jasmonate |
| MS | Murashige and Skoog medium |
| SH | Schenk and Hildebrandt medium |
References
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [PubMed]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol a stilbenic compound from grapevines against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Paulo, L.; Oleastro, M.; Eugenia Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial properties of resveratrol: A review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Research Center: Badajoz, Spain, 2015; pp. 1225–1237. [Google Scholar]
- Pezzuto, J.M. The phenomenon of resveratrol: Redefining the virtues of promiscuity. Ann. N. Y. Acad. Sci. 2001, 1215, 123–130. [Google Scholar] [CrossRef]
- Santos, J.A.; de Carvaho, G.S.G.; Oliveira, V.; Raposo, N.R.B.; da Silva, A.D. Resveratrol and analogues: A review of antioxidant activity and applications to human health. Recent Pat. Food Nutr. Agric. 2013, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Aluyen, J.K.; Ton, Q.N.; Tran, T.; Yang, A.E.; Gottlieb, H.B.; Bellanger, R.A. Resveratrol: Potential as anticancer agent. J. Diet. Suppl. 2012, 9, 45–56. [Google Scholar] [PubMed]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; Wei, X.; Shi, Z.; Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; et al. Resveratrol oligomers for the prevention and treatment of cancers resveratrol oligomers for the prevention and treatment of cancers. Oxidative Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef]
- Giovannelli, L.; Innocenti, M.; Santamaria, A.R.; Bigagli, E.; Pasqua, G.; Mulinacci, N. Antitumoural activity of viniferin-enriched extracts from Vitis vinifera L. cell cultures. Nat. Prod. Res. 2014, 28, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.T.; Zhou, Z.; Rosen, R.T. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Lamuela-Raventós, R.M. The occurrence of piceid, a stilbene glucoside in grape berries. Phytochemistry 1994, 37, 571–573. [Google Scholar] [CrossRef]
- Lu, D. Relevant enzymes, genes and regulation mechanisms in biosynthesis pathway of stilbenes. Open J. Med. Chem. 2012, 2, 15–23. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene resveratrol. J. Biomed. Biotechnol. 2012, 2012, 579089. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Tisserant, L.P.; Crouzet, J.; Courot, E. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. C. R. Chim. 2016, 19, 1062–1070. [Google Scholar]
- Georgiev, M.I.; Pavlov, A.I.; Bley, T. Hairy root type plant in vitro systems as sources of bioactive substances. Appl. Microbiol. Biotechnol. 2007, 74, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Narasu, M.L. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Nopo-Olazabal, C.; Hubstenberger, J.; Nopo-Olazabal, L.; Medina-Bolivar, F. Antioxidant activity of selected stilbenoids and their bioproduction in hairy root cultures of Muscadine grape (Vitis rotundifolia Michx.). J. Agric. Food Chem. 2013, 61, 11744–11758. [Google Scholar] [CrossRef] [PubMed]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S.; et al. Structural, functional and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, A.D.; Sahli, R.; Bisson, J.; Rivière, C.; Delaunay, J.C.; Richard, T.; Gomès, E.; Bordenave, L.; Waffo-Téguo, P.; Mérillon, J.M. Stilbenoid profiles of canes from Vitis and Muscadinia species. J. Agric. Food Chem. 2013, 61, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.M.; Cluzet, S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4′-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Jittayasothorn, Y.; Yang, Y.; Chen, S.; Wang, X.; Zhong, Y. Influences of Agrobacterium rhizogenes strains, plant genotypes, and tissue types on the induction of transgenic hairy roots in Vitis species. Vitis 2011, 50, 107–114. [Google Scholar]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, R.S.; Bourgeois, Y.; Brown, S.; Vasseur, G.; Sangwan-Norreel, B. Characterization of competent cells and early events of Agrobacterium-mediated genetic transformation in Arabidopsis thaliana. Planta 1992, 188, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Krens, F.A.; Trifonova, A.; Keizer, L.C.P.; Hall, R.D. The effect of exogenously-applied phytohormones on gene transfer efficiency in sugarbeet (Beta vulgaris L.). Plant Sci. 1996, 116, 97–106. [Google Scholar] [CrossRef]
- Do, C.B.; Cormier, F. Accumulation of anthocyanins enhanced by a high osmotic potential in grape (Vitis vinifera L.) cell suspensions. Plant Cell Rep. 1990, 9, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, K.; Yang, H.R.; Wen, P.F.; Zhang, P.; Wang, H.L.; Huang, W.D. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol. Biochem. 2010, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Belchí-Navarro, S.; Sabater-Jara, A.B.; Vera-Urbina, J.C.; Selles-Marchart, S.; Bru, R.; Pedreno, M.A. Handbook of Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin, Germany, 2013; pp. 1683–1713. [Google Scholar]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteinsstilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Kim, K.H.; Antoine, S.; Conreux, A.; de Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stirred bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Krisa, S.; Larronde, F.; Budzinski, H.; Decendit, A.; Deffieux, G.; Mérillon, J.M. Stilbene production by Vitis vinifera cell suspension cultures: Methyl jasmonate induction and 13C biolabeling. J. Nat. Prod. 1999, 62, 1688–1690. [Google Scholar] [CrossRef]
- Martinez-Esteso, M.J.; Sellés-Marchart, S.; Vera-Urbina, J.C.; Pedreño, M.A.; Bru-Martinez, R. Changes of defense proteins in the extracellular proteome of grapevine (Vitis vinifera cv Gamay) cell cultures in response to elicitors. J. Proteom. 2009, 73, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.R.; Innocenti, M.; Mulinacci, N.; Melani, F.; Valletta, A.; Sciandra, I.; Pasqua, G. Enhancement of viniferin production in Vitis vinifera L. cv. Alphonse Lavallée cell suspensions by low-energy ultrasound alone and in combination with methyl jasmonate. J. Agric. Food Chem. 2012, 60, 11135–11142. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Fornalè, S.; Franceschetti, M.; Musiani, F.; Michael, A.J.; Perry, B.; Bagni, N. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 2005, 166, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhan, J.C.; Huang, W.D. Effects of ultraviolet C, methyl jasmonate and salicylic acid alone or in combination on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. 2015, 122, 197–211. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, W.; Deng, M. Hyper-production of 13C-labeled trans-resveratrol in Vitis vinifera suspension cell culture by elicitation and in situ adsorption. Biochem. Eng. J. 2011, 53, 292–296. [Google Scholar] [CrossRef]
- Santamaria, A.R.; Antonacci, D.; Caruso, G.; Cavaliere, C.; Gubbiotti, R.; Laganà, A.; Valletta, A.; Pasqua, G. Stilbene production in cell cultures of Vitis vinifera L. cvs Globe and Michele Palieri elicited by methyl jasmonate. Nat. Prod. Res. 2010, 24, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Kastell, A.; Smetanska, I.; Ulrichs, C.; Cai, Z.; Mewis, I. Effects of phytohormones and jasmonic acid on glucosinolate content in hairy root cultures of Sinapis alba and Brassica rapa. Appl. Biochem. Biotechnol. 2013, 169, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Belchí-Navarro, S.; Martínez-Márquez, A.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martinez-Zapater, J.M.; Bru, R.; Pedreno, M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Vera-Urbina, J.C.; Selles-Marchart, S.; Martinez-Esteso, M.J.; Pedreno, M.A.; Bru-Martinez, R. Resveratrol: Source Production and Health Benefits; Delmas, D., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2013; pp. 19–40. [Google Scholar]
- Zamboni, A.; Vrhovsek, U.; Kassemeyer, H.H.; Mattivi, F.; Velasco, R. Elicitor-induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis 2006, 45, 63–68. [Google Scholar]
- Belchí-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernández-Pérez, F.; Bru, R.; Pedreño, M.A. Induction of trans-resveratrol and extracellular pathogenesis-related proteins in elicited suspension cultured cells of Vitis vinifera cv Monastrell. J. Plant Physiol. 2013, 170, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Repka, V.; Fischerová, I.; Šilhárová, K. Methyl jasmonate is a potent elicitor of multiple defense responses in grapevine leaves and cell-suspension cultures. Biol. Plant. 2004, 48, 273–283. [Google Scholar] [CrossRef]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Figueiras, A.; Gallardo, E.; Nerín, C.; Domingues, F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2014, 145, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Bru-Martinez, R.; Pedreno, M.A. Method for the Production of Resveratrol in Cell Cultures. U.S. Patent 20060205049 A1, 14 September 2006. [Google Scholar]
- Hooykaas, P.J.J.; Klapwijk, P.M.; Nuti, M.P.; Schilperoort, R.A.; Rörsch, A. Transfer of the Agrobacterium tumefaciens TI plasmid to avirulent Agrobacteria and to Rhizobium ex planta. Microbiology 1977, 98, 477–484. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Ruslan, K.; Selfitri, A.D.; Bulan, S.A.; Rukayadi, Y.; Elfahmi, T. Effect of Agrobacterium rhizogenes and elicitation on the asiaticoside production in cell cultures of Centella asiatica. Pharmacogn. Mag. 2012, 8, 111–115. [Google Scholar] [PubMed]
- Yang, D.C.; Choi, Y.E. Production of transgenic plants via Agrobacterium rhizogenes-mediated transformation of Panax ginseng. Plant Cell Rep. 2000, 19, 491–496. [Google Scholar] [CrossRef]
- Huet, Y.; Ekouna, J.P.E.; Caron, A.; Mezreb, K.; Boitel-Conti, M.; Guerineau, F. Production and secretion of a heterologous protein by turnip hairy roots with superiority over tobacco hairy roots. Biotechnol. Lett. 2013, 36, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel Kalmia latifolia by use of shoot-tip culture. Comb. Proc. Int. Plant Propag. Soc. USA 1980, 30, 421–426. [Google Scholar]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Mairet, F.; Sierra, J.; Glorian, V.; Villon, P.; Shakourzadeh, K.; Boitel-Conti, M. A new approach to define optimized range of medium composition for enhancement of hairy root production in fed-batch process. Bioprocess Biosyst. Eng. 2008, 3, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A. Pseudomonas fluorescens PTA-CT2 triggers local and systemic immune response against Botrytis cinerea in grapevine. Mol. Plant-Microbe Interact. 2015, 28, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, S.; Trotel-Aziz, P.; Villaume, S.; Couderchet, M.; Clément, C.; Aziz, A. Osmotic stress-induced polyamine oxidation mediates defence responses and reduces stress-enhanced grapevine susceptibility to Botrytis cinerea. J. Exp. Bot. 2014, 65, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, B.; Trotel-Aziz, P.; Jeandet, P.; Baillieul, F.; Aziz, A. Improved resistance against Botrytis cinerea by grapevine-associated bacteria that induce a prime oxidative burst and phytoalexin production. Phytopathology 2011, 101, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, L.P.; Hubert, J.; Lequart, M.; Borie, N.; Maurin, N.; Pilard, S.; Jeandet, P.; Aziz, A.; Renault, J.H.; Nuzilllard, J.M.; et al. 13C RNM and LC-MS Chemical profiling of major stilbenes of pharmaceutical significance produced by elicited Vitis vinifera cv. Pinot noir hairy root cultures. J. Nat. Prod. 2016, 79, 2846–2855. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, G.; Yu, K.W.; Paek, K.Y. Production of biomass and ginsenosides from adventitious roots of Panax ginseng in bioreactor cultures. Eng. Life Sci. 2005, 5, 333–342. [Google Scholar] [CrossRef]
- Sample Availability: Not available.

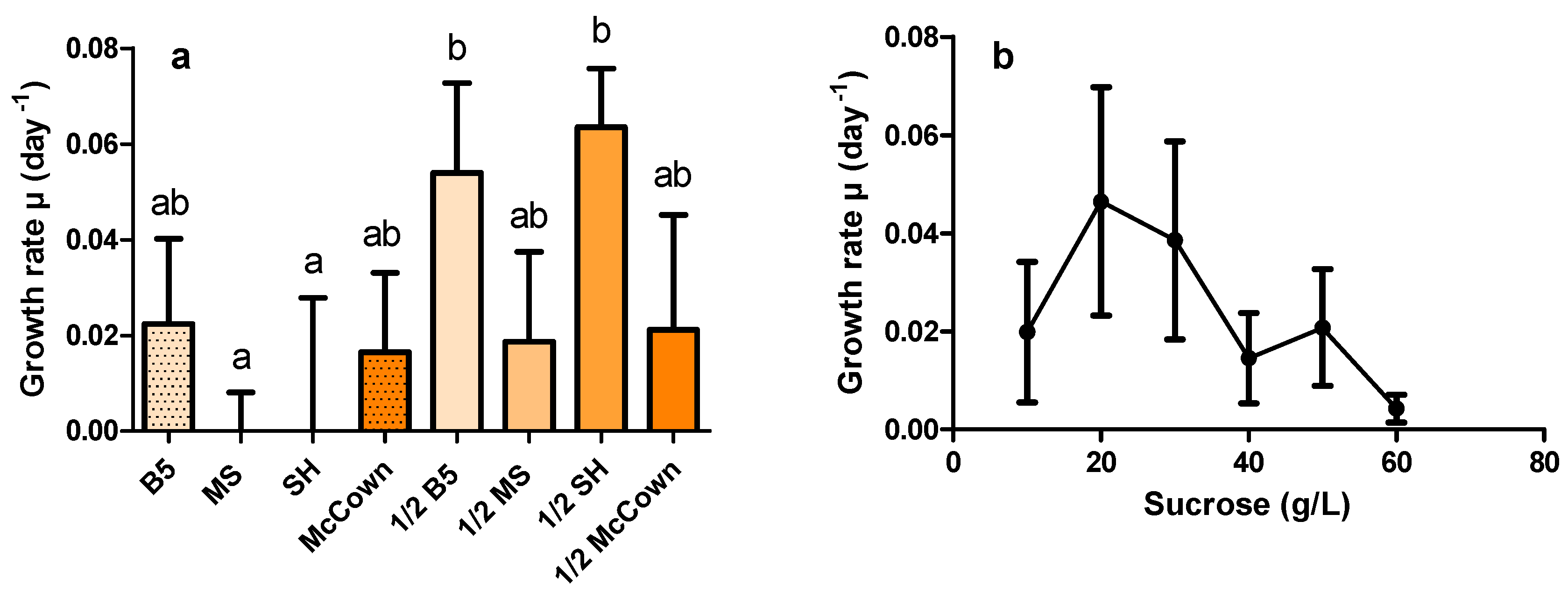

 ); ε-vin: ε-viniferin (
); ε-vin: ε-viniferin (  ); δ-vin: δ-viniferin (
); δ-vin: δ-viniferin (  ); Total (○) (sum of previous stilbenes). Each point represents the mean with standard deviation of three biological replicates.
); Total (○) (sum of previous stilbenes). Each point represents the mean with standard deviation of three biological replicates.
 ); ε-vin: ε-viniferin (
); ε-vin: ε-viniferin (  ); δ-vin: δ-viniferin (
); δ-vin: δ-viniferin (  ); Total (○) (sum of previous stilbenes). Each point represents the mean with standard deviation of three biological replicates.
); Total (○) (sum of previous stilbenes). Each point represents the mean with standard deviation of three biological replicates.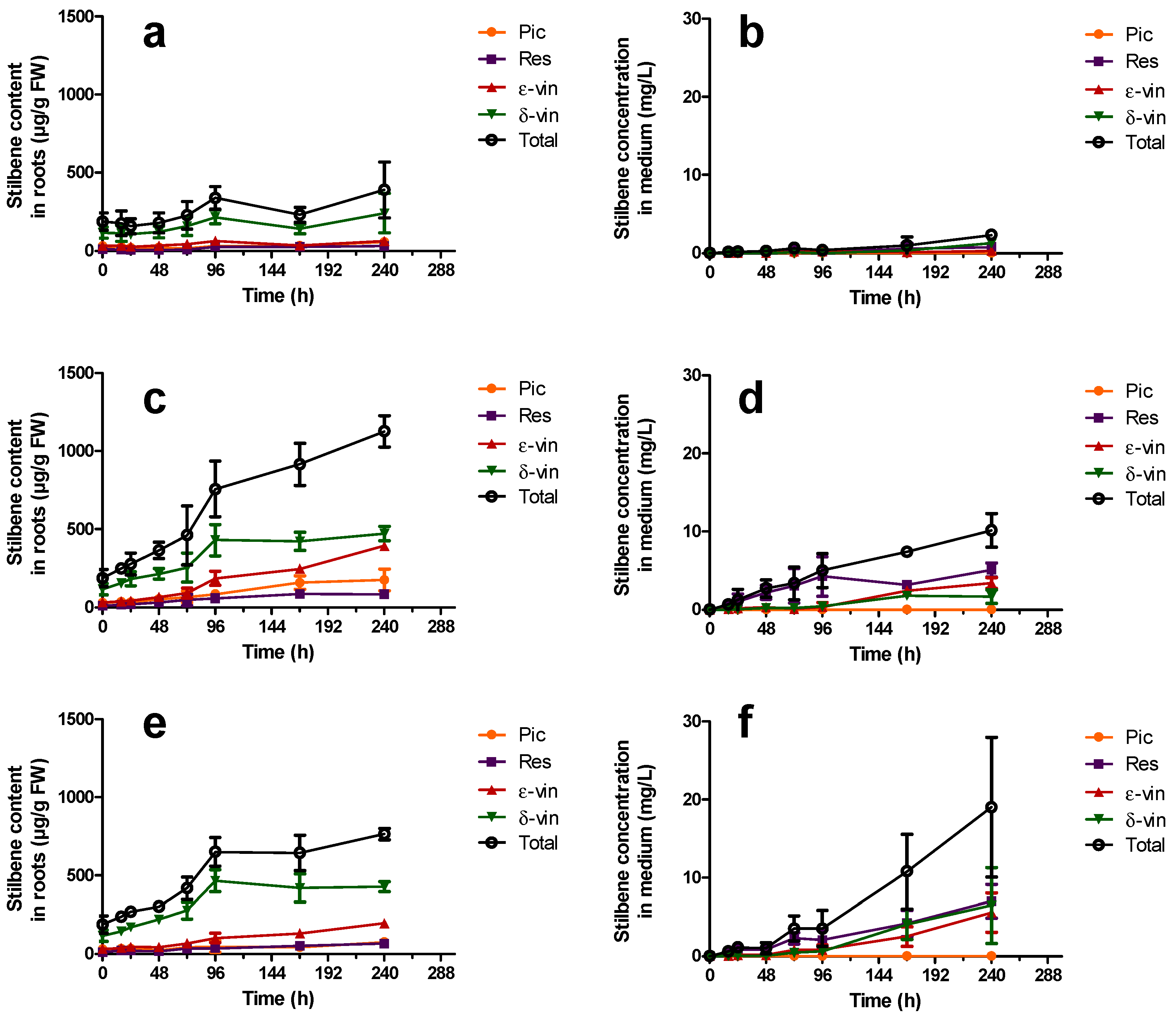
 ) or 200 µM MeJA (
) or 200 µM MeJA (  ). Each point represents the mean with standard deviation of three biological replicates.
). Each point represents the mean with standard deviation of three biological replicates.
 ) or 200 µM MeJA (
) or 200 µM MeJA (  ). Each point represents the mean with standard deviation of three biological replicates.
). Each point represents the mean with standard deviation of three biological replicates.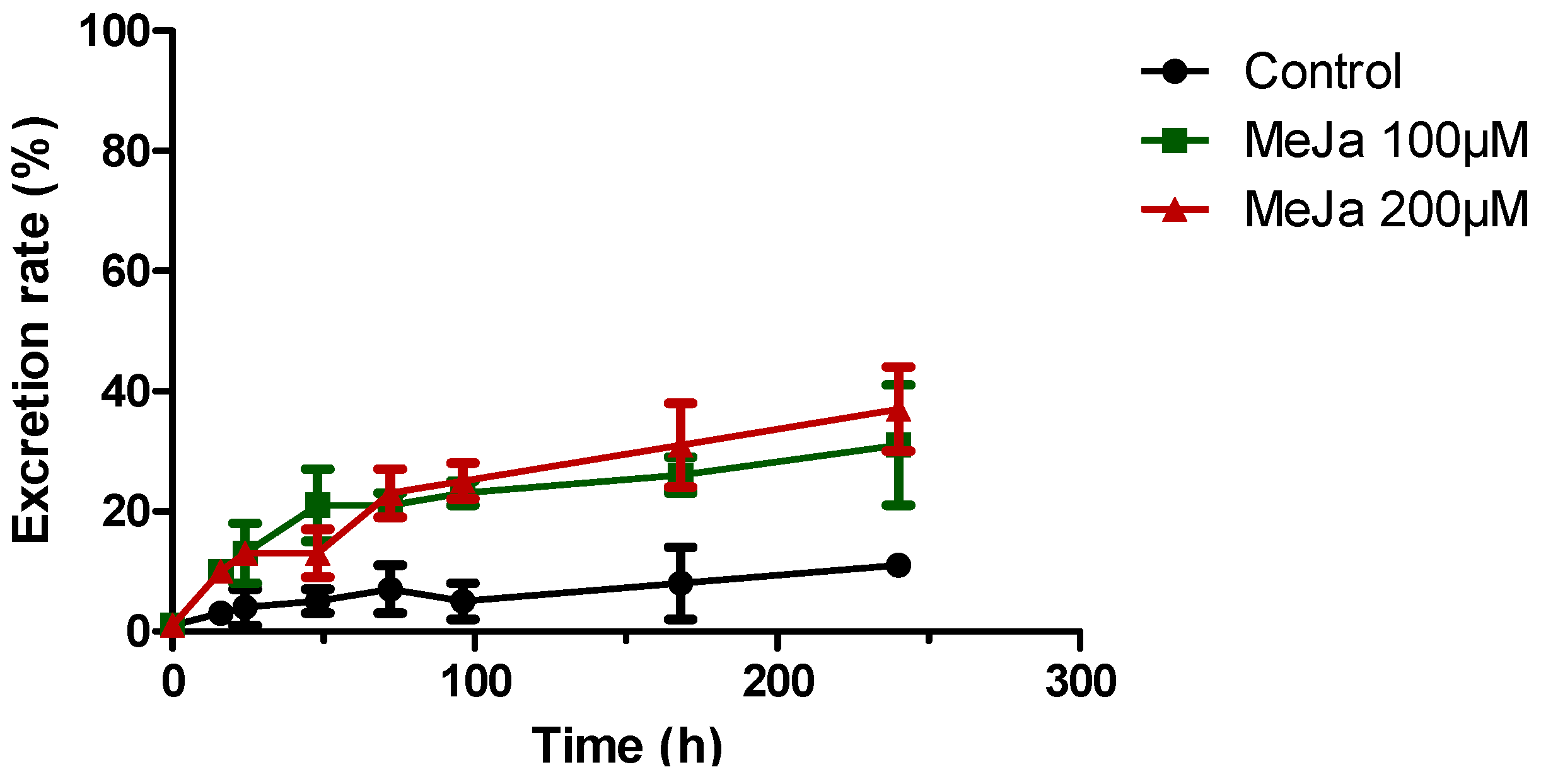
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisserant, L.-P.; Aziz, A.; Jullian, N.; Jeandet, P.; Clément, C.; Courot, E.; Boitel-Conti, M. Enhanced Stilbene Production and Excretion in Vitis vinifera cv Pinot Noir Hairy Root Cultures. Molecules 2016, 21, 1703. https://doi.org/10.3390/molecules21121703
Tisserant L-P, Aziz A, Jullian N, Jeandet P, Clément C, Courot E, Boitel-Conti M. Enhanced Stilbene Production and Excretion in Vitis vinifera cv Pinot Noir Hairy Root Cultures. Molecules. 2016; 21(12):1703. https://doi.org/10.3390/molecules21121703
Chicago/Turabian StyleTisserant, Leo-Paul, Aziz Aziz, Nathalie Jullian, Philippe Jeandet, Christophe Clément, Eric Courot, and Michèle Boitel-Conti. 2016. "Enhanced Stilbene Production and Excretion in Vitis vinifera cv Pinot Noir Hairy Root Cultures" Molecules 21, no. 12: 1703. https://doi.org/10.3390/molecules21121703







