Porphyrin Macrocycle Modification: Pyrrole Ring-Contracted or -Expanded Porphyrinoids
Abstract
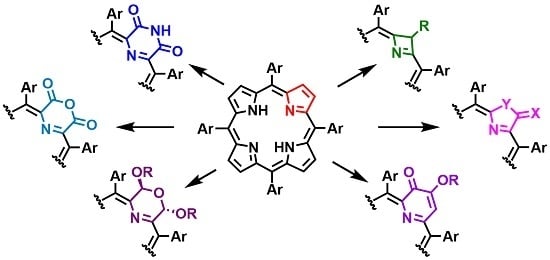
:1. Introduction
2. Chemistry
2.1. From N-Substituted Porphyrins
2.2. From β-Aminoporphyrins
2.3. From 2,3-Dihydroxychlorins
2.4. From 2,3-Dioxochlorins
2.5. From 2-Diazo-3-Oxochlorins
2.6. From Octaethyl-2-oxochlorins
2.7. From 2,3,12,13-Tetrabromoporphyrins
2.8. From β-Unsubstituted Porphyrins
2.9. Modification of Other Porphyrinoids
3. Applications
3.1. Photodynamic Therapy
3.2. Multimodal Imaging Contrast Agents
3.3. Catalysis
3.4. Optical Sensors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | benzeneselenic anhydride |
| CTAP | cetyltrimethylammonium permanganate |
| DDQ | 2,3-dichloro-5,6-dicyano-1,4-benzoquinone |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| PDT | photodynamic therapy |
| Pic | 2-picolinic acid |
| PLGA | poly(lactic-co-glycolic acid) |
| ROS | reactive oxygen species |
| TPP | meso-tetraphenylporphyrin |
| H2F20TPP | meso-tetrakis(pentafluorophenyl)porphyrin |
| p-TSA | p-toluenesulfonic acid |
References and Notes
- Srivatsan, A.; Missert, J.R.; Upadhyay, S.K.; Pandey, R.K. Porphyrin-based photosensitizers and the corresponding multifunctional nanoplatforms for cancer-imaging and phototherapy. J. Porphyr. Phthalocyanines 2015, 19, 109–134. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, A.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Porphyrins as antimicrobial photosensitizing agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Hamblin, M.R., Jori, G., Eds.; Royal Society of Chemistry: London, UK, 2011; pp. 83–160. [Google Scholar]
- Costentin, C.; Robert, M.; Savéant, J.-M. Current issues in molecular catalysis illustrated by iron porphyrins as catalysts of the CO2-to-CO electrochemical conversion. Acc. Chem. Res. 2015, 48, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tanaka, T.; Osuka, A. Fused porphyrinoids as promising near-infrared absorbing dyes. J. Mater. Chem. C 2013, 1, 2500–2519. [Google Scholar] [CrossRef]
- Goslinski, T.; Piskorz, J.J. Fluorinated porphyrinoids and their biomedical applications. Photochem. Photobiol. C 2011, 12, 304–321. [Google Scholar] [CrossRef]
- Lash, T.D. Benziporphyrins, a unique platform for exploring the aromatic characteristics of porphyrinoid systems. Org. Biomol. Chem. 2015, 13, 7846–7878. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.D. Carbaporphyrins, porphyrin isomers and the legacy of Emanuel Vogel. J. Porphyrins Phthalocyanines 2012, 16, 423–433. [Google Scholar] [CrossRef]
- Arnold, L.; Müllen, K. Modifying the porphyrin core-a chemist’s jigsaw. J. Porphyrins Phthalocyanines 2011, 15, 757–779. [Google Scholar] [CrossRef]
- Lash, T.D. Recent advances on the synthesis and chemistry of carbaporphyrins and related porphyrinoid systems. Eur. J. Org. Chem. 2007, 5461–5481. [Google Scholar] [CrossRef]
- Szyszko, B.; Latos-Grazynski, L. Core chemistry and skeletal rearrangements of porphyrinoids and metalloporphyrinoids. Chem. Soc. Rev. 2015, 44, 3588–3616. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.D. Metal complexes of carbaporphyrinoid systems. Chem. Asian J. 2014, 9, 682–705. [Google Scholar] [CrossRef] [PubMed]
- Pacholska-Dudziak, E.; Latos-Grazynski, L. Aza-deficient porphyrin as a ligand. Coord. Chem. Rev. 2009, 253, 2036–2048. [Google Scholar] [CrossRef]
- Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Porphyrins and other pyrrolic macrocycles in cycloaddition reactions. J. Porphyrins Phthalocyanines 2009, 13, 408–414. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Tomé, A.C. Cycloaddition reactions of porphyrins. Arkivoc 2003, xiv, 107–130. [Google Scholar]
- Costa, J.I.T.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. 5,10,15,20-Tetrakis(pentafluorophenyl) porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Tomé, A.C.; Lacerda, P.S.S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. meso-Arylporphyrins as dienophiles in Diels–Alder reactions: A novel approach to the synthesis of chlorins, bacteriochlorins and naphthoporphyrins. Chem. Commun. 1997, 1199–1200. [Google Scholar] [CrossRef]
- Vicente, M.G.H.; Cancilla, M.T.; Lebrilla, C.B.; Smith, K.M. Cruciform porphyrin pentamers. Chem. Commun. 1998, 2355–2356. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Novel barrelene-fused chlorins by Diels–Alder reactions. Tetrahedron Lett. 2000, 41, 3065–3068. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Kappe, C.O. Porphyrins in Diels–Alder reactions. Improvements on the synthesis of barrelene-fused chlorins using microwave irradiation. Tetrahedron Lett. 2005, 46, 4723–4726. [Google Scholar] [CrossRef]
- Zhao, S.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Cavaleiro, J.A.S.; Domingues, M.R.M.; Correia, A.J.F. Reaction of meso-tetraarylporphyrins with pyrazine ortho-quinodimethanes. Tetrahedron Lett. 2005, 46, 2189–2191. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Oliveira, K.T.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Cavaleiro, J.A.S.; Brandão, P.; Felix, V. Chemical transformations of mono- and bis(buta-1,3-dien-1-yl)porphyrins: A new synthetic approach to mono- and dibenzoporphyrins. Eur. J. Org. Chem. 2008, 704–712. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. meso-Tetraarylporphyrins as dipolarophiles in 1,3-dipolar cycloaddition reactions. Chem. Commun. 1999, 1767–1768. [Google Scholar] [CrossRef]
- Aguiar, A.; Leite, A.; Silva, A.M.N.; Tomé, A.C.; Cunha-Silva, L.; Castro, B.; Rangel, M.; Silva, A.M.G. Isoxazolidine-fused meso-tetraarylchlorins as key tools for the synthesis of mono- and bis-annulated chlorins. Org. Biomol. Chem. 2015, 13, 7131–7135. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. 1,3-Dipolar cycloaddition reactions of porphyrins with azomethine ylides. J. Org. Chem. 2005, 70, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Perrone, D.; Dondoni, A. Porphyrins in 1,3-dipolar cycloadditions with sugar azomethine ylides. Synthesis of pyrrolidinoporphyrin glycoconjugates. Synlett 2005, 857–859. [Google Scholar] [CrossRef]
- Vinhado, F.S.; Gandini, M.E.F.; Iamamoto, Y.; Silva, A.M.G.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Tomé, A.C.; Rebelo, S.L.H.; Pereira, A.M.V.M.; Cavaleiro, J.A.S. Novel Mn(III)chlorins as versatile catalysts for oxyfunctionalisation of hydrocarbons under homogeneous conditions. J. Mol. Catal. A Chem. 2005, 239, 138–143. [Google Scholar] [CrossRef]
- Galęzowski, M.; Gryko, D.T. Synthesis of locked meso-β-substituted chlorins via 1,3-dipolar cycloaddition. J. Org. Chem. 2006, 71, 5942–5950. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Porphyrins in 1,3-dipolar cycloaddition reactions: Synthesis of a novel pyrazoline-fused chlorin and a pyrazole-fused porphyrin. Synlett 2002, 71, 1155–1157. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S.; Perrone, D.; Dondoni, A. Porphyrins in 1,3-dipolar cycloaddition reactions with sugar nitrones. Synthesis of glycoconjugated isoxazolidine-fused chlorins and bacteriochlorins. Tetrahedron Lett. 2002, 43, 603–605. [Google Scholar] [CrossRef]
- Flemming, J.; Dolphin, D. Carbonyl ylide 1,3-dipolar cycloadditions with porphyrins. Tetrahedron Lett. 2002, 43, 7281–7283. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. Synthesis of new β-substituted meso-tetraphenylporphyrins via 1,3-dipolar cycloaddition reactions. 1. J. Org. Chem. 2002, 67, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, J.; Samankumara, L.P.; Brückner, C. The breaking and mending of porphyrins: Reductive coupling of secochlorin bisaldehydes. Tetrahedron Lett. 2012, 53, 3524–3526. [Google Scholar] [CrossRef]
- Boerner, L.J.K.; Dye, D.F.; Köpke, T.; Zaleski, J.M. Expansion and contraction: Shaping the porphyrin boundary via diradical reactivity. Coord. Chem. Rev. 2013, 257, 599–620. [Google Scholar] [CrossRef]
- Brückner, C.; Akhigbe, J.; Samankumara, L. Porphyrin analogs containing non-pyrrolic heterocycles. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: River Edge, NY, USA, 2014; volume 31, pp. 1–276. [Google Scholar]
- Callot, H.J.; Schaeffer, E. Homologation directe du cycle des porphyrines par les diazoalcanes. Tetrahedron 1978, 34, 2295–2300. [Google Scholar] [CrossRef]
- Callot, H.J. Ring expansions and contractions of metalloporphyrins. Dalton Trans. 2008, 6346–6357. [Google Scholar] [CrossRef] [PubMed]
- Crossley, M.J.; King, L.G. Novel heterocyclic systems from selective oxidation at the β-pyrrolic position of porphyrins. J. Chem. Soc. Chem. Commun. 1984, 920–922. [Google Scholar] [CrossRef]
- Crossley, M.J.; Hambley, T.W.; King, L.G. Conversion of a porphyrin into a 5,6-dihydroporphyrin. Synthesis and X-ray crystal structure of (5RS,6SR)-5,6-dihydro-6-(methoxycarbonyl)-8-oxo-5,10,15,20-tetraphenyl-8H-7-oxaporphyrin. Bull. Soc. Chim. Fr. 1996, 133, 735–742. [Google Scholar]
- Jayaraj, K.; Gold, A.; Austin, R.N.; Ball, L.M.; Terner, J.; Mandon, D.; Weiss, R.; Fischer, J.; DeCian, A.; Bill, E.; et al. Compound I and compound II analogues from porpholactones. Inorg. Chem. 1997, 36, 4555–4566. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.R.; Bonnett, R.; Burke, P.J.; Salgado, A.; Vallés, M.A. The 2,3-secochlorin-2,3-dione system. J. Chem. Soc. Chem. Commun. 1993, 1860–1861. [Google Scholar] [CrossRef]
- Adams, K.R.; Bonnett, R.; Burke, P.J.; Salgado, A.; Vallés, M.A. Cleavage of (octaethyl-2,3-dihydroxychlorinato)nickel(II) to give the novel 2,3-dioxo-2,3-secochlorin system. J. Chem. Soc. Perkin Trans. 1 1997, 1769–1772. [Google Scholar] [CrossRef]
- Secochlorins are porphyrin derivatives in which a β,β′-bond was cleaved; chlorophins and bacteriophins are porphyrin derivatives in which one or two sets of β,β′-carbon atoms were removed
- Ryppa, C.; Niedzwiedzki, D.; Morozowich, N.L.; Srikanth, R.; Zeller, M.; Frank, H.A.; Brückner, C. Stepwise conversion of two pyrrole moieties of octaethylporphyrin to pyridin-3-ones: Synthesis, mass spectral, and photophysical properties of mono and bis(oxypyri)porphyrins. Chem. Eur. J. 2009, 15, 5749–5762. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zeller, M.; Brückner, C. meso‑Tetraphenylporphyrin-derived oxypyriporphyrin, oxypyrichlorin, and thiomorpholinochlorin, as their Ni(II) complexes. J. Porphyr. Phthalocyanines 2012, 16, 576–588. [Google Scholar] [CrossRef]
- Brückner, C.; Rettig, S.J.; Dolphin, D. Formation of a meso-tetraphenylsecochlorin and a homoporphyrin with a twist. J. Org. Chem. 1998, 63, 2094–2098. [Google Scholar] [CrossRef]
- Brückner, C.; Sternberg, E.D.; MacAlpine, J.K.; Rettig, S.J.; Dolphin, D. A novel stepwise degradation of porphyrins. Synthesis and structural characterization of meso-tetraphenylchlorophinato nickel(II) and meso-tetraphenylsecochlorinato nickel(II). J. Am. Chem. Soc. 1999, 121, 2609–2610. [Google Scholar] [CrossRef]
- Brückner, C.; Dolphin, D. 2,3-vic-Dihydroxy-meso-tetraphenylchlorins from the osmium tetroxide oxidation of meso-tetraphenylporphyrin. Tetrahedron Lett. 1995, 36, 3295–3298. [Google Scholar] [CrossRef]
- Brückner, C.; Dolphin, D. β,β‘-Dihydroxylation of meso-tetraphenylchlorins and metallochlorins. Tetrahedron Lett. 1995, 36, 9425–9428. [Google Scholar] [CrossRef]
- Daniell, H.W.; Brückner, C. Enantiomeric resolution of a ruffled porphyrinoid. Angew. Chem. Int. Ed. 2004, 43, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Brückner, C.; Götz, D.C.G.; Fox, S.P.; Ryppa, C.; McCarthy, J.R.; Bruhn, T.; Akhigbe, J.; Banerjee, S.; Daddario, P.; Daniell, H.W.; et al. Helimeric porphyrinoids: Stereostructure and chiral resolution of meso-tetraarylmorpholinochlorins. J. Am. Chem. Soc. 2011, 133, 8740–8752. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.J.; Rusling, J.F.; Brückner, C. Nickel(II) meso-tetraphenyl-homoporphyrins, -secochlorins, and -chlorophin: Control of redox chemistry by macrocycle rigidity. J. Am. Chem. Soc. 2000, 122, 6679–6685. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Jenkins, H.A.; Brückner, C. Free base meso-tetraaryl-morpholinochlorins and porpholactone from meso-tetraaryl-2,3-dihydroxy-chlorin. Org. Lett. 2003, 5, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Khalil, G.E.; Daddario, P.; Lau, K.S.F.; Imtiaz, S.; King, M.; Gouterman, M.; Sidelev, A.; Puran, N.; Ghandehari, M.; Brückner, C. meso-Tetraarylporpholactones as high pH sensors. Analyst 2010, 135, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Brückner, C.; Ogikubo, J.; McCarthy, J.R.; Akhigbe, J.; Hyland, M.A.; Daddario, P.; Worlinsky, J.L.; Zeller, M.; Engle, J.T.; Ziegler, C.J.; et al. meso-Arylporpholactones and their reduction products. J. Org. Chem. 2012, 77, 6480–6494. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zeller, M.; Brückner, C. OsO4-mediated dihydroxylation of meso-tetraphenylporphyrin N-oxide and transformation of the resulting diolchlorin N-oxide regioisomers. J. Org. Chem. 2010, 75, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Melfi, P.J.; Capetta, S.H.; Brückner, C. Use of Ag(II) as a removable template in porphyrin chemistry: Diol cleavage products of [meso-tetraphenyl-2,3-cis-diolchlorinato]silver(II). Tetrahedron 2003, 59, 9137–9146. [Google Scholar] [CrossRef]
- Lara, K.K.; Rinaldo, C.R.; Brückner, C. meso-Tetraaryl-7,8-dihydroxydithiachlorins: First examples of heterochlorins. Tetrahedron Lett. 2003, 44, 7793–7797. [Google Scholar] [CrossRef]
- Lara, K.K.; Rinaldo, C.K.; Brückner, C. meso-Tetraaryl-7,8-diol-21,23-dithiachlorins and their pyrrole-modified derivatives: A spectroscopic comparison to their aza-analogues. Tetrahedron 2005, 61, 2529–2539. [Google Scholar] [CrossRef]
- Akhigbe, J.; Ryppa, C.; Zeller, M.; Brückner, C. Oxazolochlorins. 2. Intramolecular Cannizzaro reaction of meso-tetraphenylsecochlorin bisaldehyde. J. Org. Chem. 2009, 74, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Samankumara, L.P.; Wells, S.; Zeller, M.; Acuña, A.M.; Röder, B.; Brückner, C. Expanded bacteriochlorins. Angew. Chem. Int. Ed. 2012, 51, 5757–5760. [Google Scholar] [CrossRef] [PubMed]
- Samankumara, L.P.; Zeller, M.; Krause, J.A.; Brückner, C. Syntheses, structures, modification, and optical properties of meso-tetraaryl-2,3-dimethoxychlorin, and two isomeric meso-tetraaryl-2,3,12,13-tetrahydroxybacteriochlorins. Org. Biomol. Chem. 2010, 8, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Hyland, M.A.; Brückner, C. Indaphyrin, a meso-tetraphenylsecochlorin-derived chromophore incorporating o-phenyl-to-β-linkages. Chem. Commun. 2003, 1738–1739. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Hyland, M.A.; Brückner, C. Synthesis of indaphyrins: Meso-Tetraarylsecochlorin-based porphyrinoids containing direct o-phenyl-to-β-linkages. Org. Biomol. Chem. 2004, 2, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.F.; Zhao, S.; Ryppa, C.; Jockusch, S.; Turro, N.J.; Zeller, M.; Gouterman, M.; Khalil, G.E.; Brückner, C. Synthesis, structure, and optical properties of the platinum(II) complexes of indaphyrin and thiaindaphyrin. Inorg. Chem. 2009, 48, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
- Samankumara, L.P.; Dorazio, S.J.; Akhigbe, J.; Li, R.; Nimthong-Roldán, A.; Zeller, M.; Brückner, C. Indachlorins: Nonplanar indanone-annulated chlorin analogues with panchromatic absorption spectra between 300 and 900 nm. Chem. Eur. J. 2015, 21, 11118–11128. [Google Scholar] [CrossRef] [PubMed]
- Götz, D.C.G.; Gehrold, A.C.; Dorazio, S.J.; Daddario, P.; Samankumara, L.; Bringmann, G.; Brückner, C.; Bruhn, T. Indaphyrins and indachlorins: Optical and chiroptical properties of a family of helimeric porphyrinoids. Eur. J. Org. Chem. 2015, 18, 3913–3922. [Google Scholar] [CrossRef]
- Banerjee, S.; Hyland, M.A.; Brückner, C. [2-Methylazeteochlorinato]Ni(II): A pyrrole ring-contracted chlorin analogue. Tetrahedron Lett. 2010, 51, 4505–4508. [Google Scholar] [CrossRef]
- Brückner, C.; Hyland, M.A.; Sternberg, E.D.; MacAlpine, J.K.; Rettig, S.J.; Patrick, B.O.; Dolphin, D. Preparation of [meso-tetraphenylchlorophinato]nickel(II) by stepwise deformylation of [meso-tetraphenyl-2,3-diformyl-secochlorinato]nickel(II): Conformational consequences of breaking the structural integrity of nickel porphyrins. Inorg. Chim. Acta 2005, 358, 2943–2953. [Google Scholar] [CrossRef]
- Köpke, T.; Pink, M.; Zaleski, J.M. Elucidation of the extraordinary 4-membered pyrrole ring-contracted azeteoporphyrinoid as an intermediate in chlorin oxidation. Chem. Commun. 2006, 4940–4942. [Google Scholar] [CrossRef]
- Banerjee, S.; Zeller, M.; Brückner, C. MTO/H2O2/pyrazole-mediated N-oxidation of meso-tetraarylporphyrins and -chlorins, and S-oxidation of a meso-tetraaryldithiaporphyrin and -chlorin. J. Org. Chem. 2009, 74, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Kozyrev, A.N.; Alderfer, J.L.; Dougherty, T.J.; Pandey, R.K. Synthesis of mono- and di(oxopyri)porphyrins: a new approach through ring enlargement with diazomethane. Angew. Chem. Int. Ed. 1999, 38, 126–128. [Google Scholar] [CrossRef]
- Akhigbe, J.; Brückner, C. Expansion of a pyrrole in meso-tetraphenylporphyrin to a pyrazine imide moiety using a Beckmann rearrangement. Eur. J. Org. Chem. 2013, 3876–3884. [Google Scholar] [CrossRef]
- Akhigbe, J.; Zeller, M.; Brückner, C. Quinoline-annulated porphyrins. Org. Lett. 2011, 13, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, J.; Luciano, M.; Zeller, M.; Brückner, C. Mono- and bisquinoline-annulated porphyrins from porphyrin β,β′‑dione oximes. J. Org. Chem. 2015, 80, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, J.A.S.; Gerdan, V.M.; Hombrecher, H.K.; Neves, M.G.P.M.S.; Silva, A.M.S. Synthesis and characterisation of new 2-diazo-3-oxo-5,10,15,20-tetraphenylchlorins. Heterocycl. Commun. 1997, 3, 253–261. [Google Scholar] [CrossRef]
- Köpke, T.; Pink, M.; Zaleski, J.M. A facile synthesis of 2-diazo-3-oxochlorins by lewis acid activation: selective modification of π-electron conjugated macrocycles. Synlett 2006, 2183–2186. [Google Scholar] [CrossRef]
- Hombrecher, H.K.; Gerdan, V.M.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S. Photoinduced reaction of 2-diazo-3-oxo-5,10,15,20-tetraphenylchlorins with alcohols. Heterocycl. Commun. 1997, 3, 453–460. [Google Scholar] [CrossRef]
- Köpke, T.; Pink, M.; Zaleski, J.M. Photochemical preparation of pyrrole ring-contracted chlorins by the Wolff rearrangement. Org. Biomol. Chem. 2006, 4, 4059–4062. [Google Scholar] [CrossRef] [PubMed]
- Köpke, T.; Pink, M.; Zaleski, J.M. Expansion by contraction: Diversifying the photochemical reactivity scope of diazo-oxochlorins toward development of in situ alkylating agents. J. Am. Chem. Soc. 2008, 130, 15864–15871. [Google Scholar] [CrossRef] [PubMed]
- Kopke, T.; Zaleski, J.M. Diazo-containing molecular constructs as potential anticancer agents: From diazo[b]fluorene natural products to photoactivatable diazo-oxochlorins. Anti-Cancer Agents Med. Chem. 2008, 8, 292–304. [Google Scholar] [CrossRef]
- Dye, D.F.; Köpke, T.; Ramabhadran, R.O.; Raghavachari, K.; Zaleski, J.M. Gating the mechanistic pathway to the elusive 4-membered ring azeteoporphyrin. J. Am. Chem. Soc. 2011, 133, 13110–13120. [Google Scholar] [CrossRef] [PubMed]
- Meehan, E.; Li, R.; Zeller, M.; Brückner, C. Octaethyl-1,3-oxazinochlorin: A β‑octaethylchlorin analogue made by pyrrole expansion. Org. Lett. 2015, 17, 2210–2213. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-L.; Guo, C.-C.; Chen, Q.-Y. Unprecedented degradation of nickel(II) 2,3,12,13-tetrabromo-5,10,15,20-tetraarylporphyrins by the anion of E-benzaldoxime: A novel approach to nickel(II) chlorophins and bacteriophins. Org. Lett. 2010, 11, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.M.; Biteva, I.M.; Gurinovich, I.F.; Grubina, L.A.; Gurinovich, G.P. Oxidation of porphyrins—Structure of products of octaethylporphin ozonization. Biofisika 1977, 22, 771–776. [Google Scholar]
- Gouterman, M.; Hall, R.J.; Khalil, G.-E.; Martin, P.C.; Shankland, E.G.; Cerny, R.L. Tetra(pentafluorophenyl)porpholactone. J. Am. Chem. Soc. 1989, 111, 3702–3707. [Google Scholar] [CrossRef]
- Khalil, G.; Gouterman, M.; Ching, S.; Costin, C.; Coyle, L.; Gouin, S.; Green, E.; Sadilek, M.; Wan, R.; Yearyean, J.; et al. Synthesis and spectroscopic characterization of Ni, Zn, Pd and Pt tetra(pentafluorophenyl)porpholactone with comparisons to Mg, Zn, Y, Pd and Pt metal complexes of tetra(pentafluorophenyl)porphine. J. Porphyr. Phthalocyanines 2002, 6, 135–145. [Google Scholar] [CrossRef]
- Lv, H.; Yang, B.; Jing, J.; Yu, Y.; Zhang, J.; Zhang, J.-L. Dual facet of gold(III) in the reactions of gold(III) and porphyrins. Dalton Trans. 2012, 41, 3116–3118. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, H.; Ke, X.; Yang, B.; Zhang, J.-L. Ruthenium-catalyzed oxidation of the porphyrin β-β’-pyrrolic ring: A general and efficient approach to porpholactones. Adv. Synth. Catal. 2012, 354, 3509–3516. [Google Scholar] [CrossRef]
- Ke, X.-S.; Chang, Y.; Chen, J.-Z.; Tian, J.; Mack, J.; Cheng, X.; Shen, Z.; Zhang, J.-L. Porphodilactones as synthetic chlorophylls: Relative orientation of β‑substituents on a pyrrolic ring tunes NIR absorption. J. Am. Chem. Soc. 2014, 136, 9598–9607. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, J.; Peters, G.; Zeller, M.; Brückner, C. Unexpected hydroxylamine-induced ring-closure reactions of meso-tetraphenylsecochlorin bisaldehyde. Org. Biomol. Chem. 2011, 9, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, J.; Haskoor, J.; Zeller, M.; Brückner, C. Porpholactams and chlorolactams: Replacement of a β-β’-double bond in meso-tetraphenyl-porphyrin and -chlorin by a lactam moiety. Chem. Commun. 2011, 47, 8599–8601. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, J.; Haskoor, J.; Krause, J.A.; Zeller, M.; Brückner, C. Formation, structure, and reactivity of meso-tetraarylchlorolactones, -porpholactams, and -chlorolactams, porphyrin and chlorin analogues incorporating oxazolone or imidazolone moieties. Org. Biomol. Chem. 2013, 11, 3616–3628. [Google Scholar] [CrossRef] [PubMed]
- Brückner, C.; McCarthy, J.R.; Daniell, H.W.; Pendon, Z.D.; Ilagan, R.P.; Francis, T.M.; Ren, L.; Birge, R.R.; Frank, H.A. A spectroscopic and computational study of the singlet and triplet excited states of synthetic β-functionalized chlorins. Chem. Phys. 2003, 294, 285–303. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Perez, M.J.; Brückner, C.; Weissleder, R. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 2005, 5, 2552–2556. [Google Scholar] [CrossRef] [PubMed]
- Ogikubo, J.; Worlinsky, J.L.; Fu, Y.-J.; Brückner, C. A two-step, one-pot route to swap the pyrroline moiety in meso-tetraaryldihydroxy-chlorins with an O/N-substituted oxazoline. Tetrahedron Lett. 2013, 54, 1707–1710. [Google Scholar] [CrossRef]
- Ogikubo, J.; Brückner, C. Tunable meso-tetraphenyl-alkyloxazolo-chlorins and –bacteriochlorins. Org. Lett. 2011, 13, 2380–2383. [Google Scholar] [CrossRef] [PubMed]
- Ogikubo, J.; Meehan, E.; Engle, J.T.; Ziegler, C.J.; Brückner, C. meso-Aryl-3-alkyl-2-oxachlorins. J. Org. Chem. 2012, 77, 6199–6207. [Google Scholar] [CrossRef] [PubMed]
- Ogikubo, J.; Meehan, E.; Engle, J.T.; Ziegler, C.J.; Brückner, C. Oxazolochlorins. 9. meso-Tetraphenyl-2-oxabacteriochlorins and meso-tetraphenyl-2,12/13-dioxabacteriochlorins. J. Org. Chem. 2013, 78, 2840–2852. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Czepukojc, B.; Jacob, C.; Jiang, Y.; Zeller, M.; Brückner, C.; Zhang, J.-L. Porphothionolactones: Synthesis, structure, physical, and chemical properties of a chemodosimeter for hypochlorite. Org. Biomol. Chem. 2013, 11, 4613–4621. [Google Scholar] [CrossRef] [PubMed]
- Head, M.L.; Zarate, G.; Brückner, C. Pyrazinoporphyrins: Expanding a pyrrolic building block in meso‑tetraphenylporphyrin by a nitrogen atom. Eur. J. Org. Chem. 2016, 992–998. [Google Scholar] [CrossRef]
- Ke, X.-S.; Tang, J.; Chen, J.-J.; Zhou, Z.-Y.; Zhang, J.-L. β-Ionic conjugated chlorin-type photosensitizers based on porpholactone: Synthesis, photophysical properties, and photodynamic activity. ChemPlusChem. 2015, 80, 237–252. [Google Scholar] [CrossRef]
- Tang, J.; Chen, J.-J.; Jing, J.; Chen, J.-Z.; Lv, H.; Yu, Y.; Xu, P.; Zhang, J.-L. β-Lactonization of fluorinated porphyrin enhances LDL binding affinity, cellular uptake with selective intracellular localization. Chem. Sci. 2014, 5, 558–566. [Google Scholar] [CrossRef]
- Ke, X.-S.; Tang, J.; Yang, Z.-S.; Zhang, J.-L. β-Conjugation of gadolinium(III) DOTA complexes to zinc(II) porpholactol as potential multimodal imaging contrast agents. J. Porphyr. Phthalocyanines 2014, 18, 950–959. [Google Scholar] [CrossRef]
- Cetin, A.; Ziegler, C.J. Structure and catalytic activity of a manganese(III) tetraphenylporpholactone. Dalton Trans. 2005, 25–26. [Google Scholar] [CrossRef]
- Rahimi, R.; Tehrani, A.A.; Fard, M.A.; Sadegh, B.M.M.; Khavasi, H.R. First catalytic application of metal complexes of porpholactone and dihydroxychlorin in the sulfoxidation reaction. Catal. Commun. 2009, 11, 232–235. [Google Scholar] [CrossRef]
- Liang, L.; Lv, H.; Yu, Y.; Wang, P.; Zhang, J.-L. Iron(III) tetrakis(pentafluorophenyl)porpholactone catalyzes nitrogen atom transfer to C=C and C–H bonds with organic azides. Dalton Trans. 2012, 41, 1457. [Google Scholar] [CrossRef] [PubMed]
- To, W.-P.; Liu, Y.; Lau, T.-C.; Che, C.-M. A robust palladium(II)–porphyrin complex as catalyst for visible light induced oxidative C–H functionalization. Chem. Eur. J. 2013, 19, 5654–5664. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Labuta, J.; van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Zelelow, B.; Khalil, G.E.; Phelan, G.; Carlson, B.; Gouterman, M.; Callis, J.B.; Dalton, L.R. Dual luminophor pressure sensitive paint. II. Lifetime based measurement of pressure and temperature. Sens. Actuators B 2003, 96, 304–314. [Google Scholar] [CrossRef]
- Khalil, G.E.; Costin, C.; Crafton, J.; Jones, G.; Grenoble, S.; Gouterman, M.; Callis, J.B.; Dalton, L.R. Dual-luminophor pressure-sensitive paint. I. Ratio of reference to sensor giving a small temperature dependency. Sens. Actuators B 2004, 97, 13–21. [Google Scholar] [CrossRef]
- Gouterman, M.; Callis, J.; Dalton, L.; Khalil, G.; Mébarki, Y.; Cooper, K.R.; Grenier, M. Dual luminophor pressure-sensitive paint: III. Application to automotive model testing. Meas. Sci. Technol. 2004, 15, 1986–1994. [Google Scholar] [CrossRef]
- Waskitoaji, W.; Hyakutake, T.; Watanabe, M.; Nishide, H. Pt-porpholactone- and -porphyrin-based luminescent sensory polymer coating for visualization of oxygen pressure distribution on biplanar surface. React. Funct. Polym. 2010, 70, 669–673. [Google Scholar] [CrossRef]
- Ishigami, Y.; Waskitoaji, W.; Yoneda, M.; Takada, K.; Hyakutake, T.; Suga, T.; Uchida, M.; Nagumo, Y.; Inukai, J.; Nishide, H.; et al. Oxygen partial pressures on gas-diffusion layer surface and gas-flow channel wall in polymer electrolyte fuel cell during power generation studied by visualization technique combined with numerical simulation. J. Power Sources 2014, 269, 556–564. [Google Scholar] [CrossRef]
- Rodrigues, J.M.M.; Farinha, A.A.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Tomé, J.P.C. Highly selective optical chemosensor for cyanide in aqueous medium. Sens. Actuators B Chem. 2016, 224, 81–87. [Google Scholar] [CrossRef]
- Worlinsky, J.L.; Halepas, S.; Brückner, C. PEGylated meso-arylporpholactone metal complexes as optical cyanide sensors in water. Org. Biomol. Chem. 2014, 12, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.-S.; Yang, B.-Y.; Cheng, X.; Chan, S.L.-F.; Zhang, J.-L. Ytterbium(III) porpholactones: β-Lactonization of porphyrin ligands enhances sensitization efficiency of lanthanide near-infrared luminescence. Chem. Eur. J. 2014, 20, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Worlinsky, J.L.; Zarate, G.; Zeller, M.; Ghandehari, M.; Khalil, G.; Brückner, C. Oxazolochlorins. 11. Tuning the dynamic high pH sensing range of [meso-tetraarylporpholactonato]M(II) complexes by variation of the central metal ion, the aryl substituents, and introduction of a β-nitro group. J. Porphyr. Phthalocyanines 2013, 17, 836–849. [Google Scholar] [CrossRef]
- Worlinsky, J.L.; Halepas, S.; Ghandehari, M.; Khalil, G.; Brückner, C. High pH sensing with water-soluble porpholactone derivatives and their incorporation into a Nafion® optode membrane. Analyst 2015, 140, 190–196. [Google Scholar] [CrossRef] [PubMed]






































| M44 | BSA | Reaction Time | M46 | M5 | M44 Recovered |
|---|---|---|---|---|---|
| Cu44 | 2 equiv | 22 h | 8% | 27% | 59% |
| Cu44 | 4 equiv | 8 h | 7% | 70% | 11% |
| Cu44 | 4 equiv | 18 h | traces | 75% | — |
| Cu44 | 8 equiv | 5 h | 6% | 82% | traces |
| Ni44 | 4 equiv | 14 h | 18% | 14% | 65% |
| Ni44 | 6 equiv | 9 h | 19% | 35% | 20% |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, L.D.; Costa, J.I.T.; Tomé, A.C. Porphyrin Macrocycle Modification: Pyrrole Ring-Contracted or -Expanded Porphyrinoids. Molecules 2016, 21, 320. https://doi.org/10.3390/molecules21030320
Costa LD, Costa JIT, Tomé AC. Porphyrin Macrocycle Modification: Pyrrole Ring-Contracted or -Expanded Porphyrinoids. Molecules. 2016; 21(3):320. https://doi.org/10.3390/molecules21030320
Chicago/Turabian StyleCosta, Letícia D., Joana I.T. Costa, and Augusto C. Tomé. 2016. "Porphyrin Macrocycle Modification: Pyrrole Ring-Contracted or -Expanded Porphyrinoids" Molecules 21, no. 3: 320. https://doi.org/10.3390/molecules21030320