Rosmarinic Acid Attenuates Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of RA on Inflammatory Cells and Th2 Cytokines in Bronchoalveolar Lavage Fluid (BALF)
2.2. Effects of RA on Total IgE, Ova-Specific IgE and Eotaxin Concentrations
2.3. Effects of RA on Histopathological Changes in Lungs
2.4. Effects of RA on AHR to Methacholine (Mch)
2.5. Effects of RA on MAPK and NF-κB Activation
2.6. Effects of RA on Inflammatory Gene Expression
2.7. Discussion
3. Material and Methods
3.1. Animals
3.2. Reagents
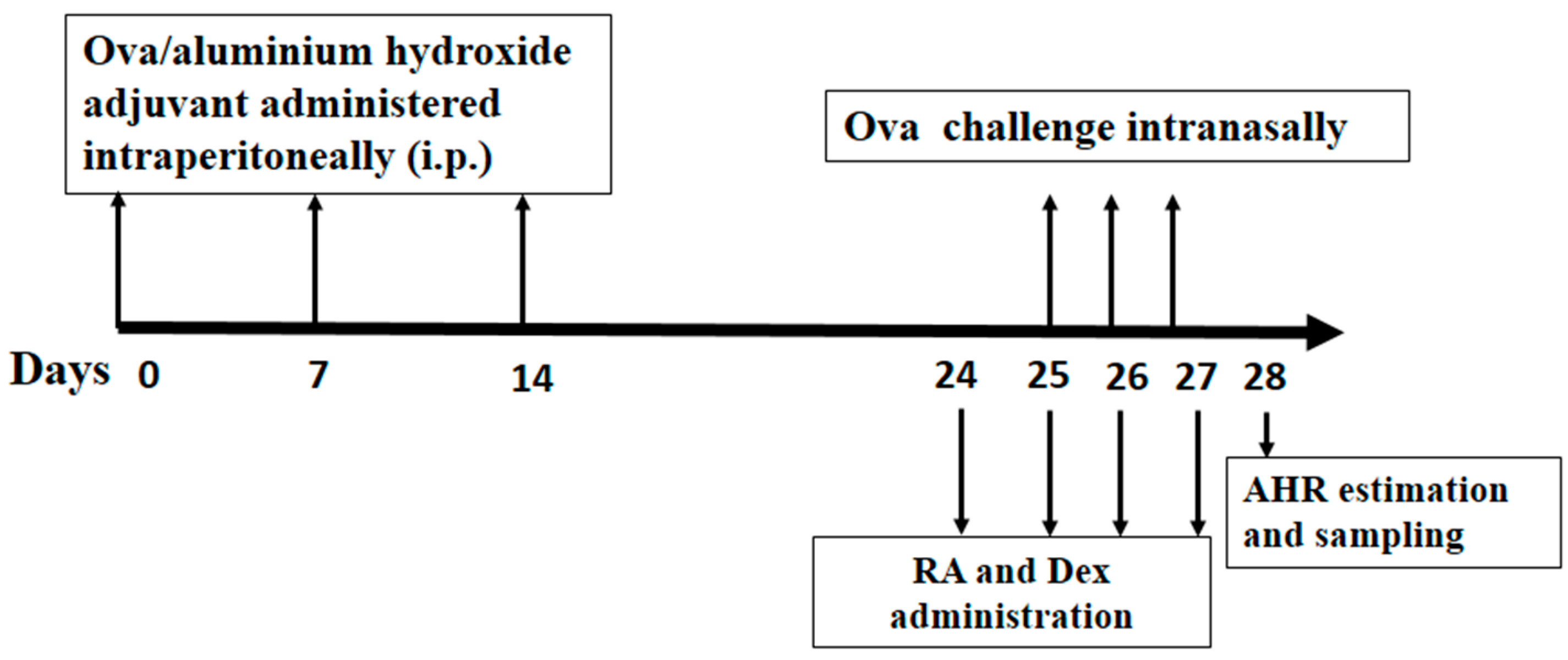
3.3. Grouping, Sensitization, Challenge and Pretreatment of Mice
3.4. Collection of Blood and BALF
3.5. Measurements of Cytokines, Chemokines and IgE Production
3.6. Histologic Analysis
3.7. Measurements of AHR
3.8. Western Blot Analysis of MAPK and NF-κB
3.9. RNA Preparation and Quantitative RT-PCR
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agarwal, R.; Gupta, D. Severe asthma and fungi: Current evidence. Med. Mycol. 2011, 49 (Suppl. S1), S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Crane, J.; Lai, C.K.; Pearce, N. Prevalence and etiology of asthma. J. Allergy Clin. Immunol. Pract. 2000, 105, S466–S472. [Google Scholar] [CrossRef]
- Desmet, C.; Gosset, P.; Pajak, B.; Cataldo, D.; Bentires-Alj, M.; Lekeux, P.; Bureau, F. Selective blockade of NF-κB activity in airway immune cells inhibits the effector phase of experimental asthma. J. Immunol. 2004, 173, 5766–5775. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Epithelial cells as immunoregulators in allergic airway diseases. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cytokines as mediators of chronic asthma. Am. J. Respir. Crit. Care Med. 1994, 150, S42–S49. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Lemanske, R.F., Jr. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [PubMed]
- Herrick, C.A.; Bottomly, K. To respond or not to respond: T cells in allergic asthma. Nat. Rev. Immunol. 2003, 3, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.-K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Durham, S. Mechanisms of mucosal inflammation in the nose and lungs. Clin. Exp. Allergy 1998, 28, 11–16. [Google Scholar] [PubMed]
- Kroegel, C.; Liu, M.C.; Hubbard, W.C.; Lichtenstein, L.M.; Bochner, B.S. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: Surface phenotype, density heterogeneity, and prostanoid production. J. Allergy Clin. Immunol. Pract. 1994, 93, 725–734. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kato, R.; Fukuyama, S.; Nonami, A.; Taniguchi, K.; Matsumoto, K.; Nakano, T.; Tsuda, M.; Matsumura, M.; Kubo, M. Spred-1 negatively regulates allergen-induced airway eosinophilia and hyperresponsiveness. J. Exp. Med. 2005, 201, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-W.; Kim, D.-K.; Ko, H.-M.; Lee, H.-K. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-κB inhibits established asthmatic reaction in mice. Int. Immunopharmacol. 2004, 4, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-L.; Pei, D.-A.; Yan, J.-Z.; Xu, G.; Wu, P. A20 Overexpression Inhibits Lipopolysaccharide-Induced NF-κB Activation, TRAF6 and CD40 Expression in Rat Peritoneal Mesothelial Cells. Int. J. Mol. Sci. 2014, 15, 6592–6608. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-H.; Shin, D.; Han, S.-Y.; Kim, J.-L.; Kang, Y.-H. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 2012, 142, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Guan, S.; Cheng, C.; Wu, S.; Wong, S.H.; Kemeny, D.M.; Leung, B.P.; Wong, W.F. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am. J. Respir. Crit. Care Med. 2009, 179, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Tsang, F.; Koh, A.H.M.; Ting, W.L.; Wong, P.T.H.; Wong, W.S.F. Effects of mitogen-activated protein kinase kinase inhibitor PD 098059 on antigen challenge of guinea-pig airways in vitro. Br. J. Pharmacol. 1998, 125, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Psotova, J.; Kolar, M.; Sousek, J.; Svagera, Z.; Vicar, J.; Ulrichova, J. Biological activities of Prunella vulgaris extract. Phytother. Res. 2003, 17, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Ci, X.; He, J.; Jiang, L.; Wei, M.; Cao, Q.; Guan, M.; Xie, X.; Deng, X.; He, J. Effects of a natural prolyl oligopeptidase inhibitor, rosmarinic acid, on lipopolysaccharide-induced acute lung injury in mice. Molecules 2012, 17, 3586–3598. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, J. The mechanisms of action of disease-modifying antirheumatic drugs: A review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen. Pharmacol. 1997, 29, 127–150. [Google Scholar] [CrossRef]
- Gagliardo, R.; Chanez, P.; Mathieu, M.; Bruno, A.; Costanzo, G.; Gougat, C.; Vachier, I.; Bousquet, J.; Bonsignore, G.; Vignola, A.M. Persistent activation of nuclear factor–κB signaling pathway in severe uncontrolled asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Cytokines and chemoattractants in allergic inflammation. Mol. Immunol. 2002, 38, 881–885. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, H.; Wong, C.H.; Leung, K.Y.; Wong, W. Increased lungkine and chitinase levels in allergic airway inflammation: A proteomics approach. Proteomics 2005, 5, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.P.; Rosenberg, H.F.; Moqbel, R.; Phipps, S.; Foster, P.S.; Lacy, P.; Kay, A.B.; Rothenberg, M.E. Eosinophils: Biological properties and role in health and disease. Clin. Exp. Allergy 2008, 38, 709–750. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ci, X.; Chu, X.; Wei, M.; Hua, S.; Deng, X. Hesperidin suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Inflammation 2012, 35, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Boyton, R.; Altmann, D. Asthma: New developments in cytokine regulation. Clin. Exp. Allergy 2004, 136, 13–14. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 1998, 8, 275–283. [Google Scholar] [CrossRef]
- Murphy, K.M.; Ouyang, W.; Farrar, J.D.; Yang, J.; Ranganath, S.; Asnagli, H.; Afkarian, M.; Murphy, T.L. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000, 18, 451–494. [Google Scholar] [CrossRef] [PubMed]
- Glimcher, L.H.; Murphy, K.M. Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes Dev. 2000, 14, 1693–1711. [Google Scholar] [PubMed]
- Vieira-de-Abreu, A.; Assis, E.F.; Gomes, G.S.; Castro-Faria-Neto, H.C.; Weller, P.F.; Bandeira-Melo, C.; Bozza, P.T. Allergic challenge-elicited lipid bodies compartmentalize in vivo leukotriene C4 synthesis within eosinophils. Am. J. Respir. Cell Mol. Biol. 2005, 33, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Yuda, H.; Adachi, Y.; Taguchi, O.; Gabazza, E.C.; Hataji, O.; Fujimoto, H.; Tamaki, S.; Nishikubo, K.; Fukudome, K.; D’Alessandro-Gabazza, C. Activated protein C inhibits bronchial hyperresponsiveness and Th2 cytokine expression in mice. Blood 2004, 103, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.-P.; Kong, L.-R.; Cheng, C.; Lim, J.C.; Wong, W.F. Protective role of 14-deoxy-11, 12-didehydroandrographolide, a noncytotoxic analogue of andrographolide, in allergic airway inflammation. J. Nat. Prod. 2011, 74, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.A.; Krishnan, V.L.; Adcock, I.M.; Barnes, P.J.; Chung, K.F. Activation and localization of transcription factor, nuclear factor-κB, in asthma. Am. J. Respir. Crit. Care Med. 1998, 158, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I. Transcription factors and asthma. Eur. Respir. J. 1998, 12, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Herbert, C.; Thomas, P.S.; Wollin, L.; Beume, R.; Yang, M.; Webb, D.C.; Foster, P.S. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J. Pharmacol. Exp. Ther. 2003, 307, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Jabara, H.H.; Geha, R.S. Jun N-terminal kinase is essential for CD40-mediated IgE class switching in B cells. J. Allergy Clin. Immunol. Pract. 2005, 115, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.L.; Lee, C.G.; Jarjour, N.; Shim, Y.M.; Holm, C.T.; He, S.; Dziura, J.D.; Reed, J.; Coyle, A.J.; Kiener, P. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007, 357, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, J.-A.; Lloyd, C.M.; Wen, D.; Albar, J.P.; Wells, T.N.; Proudfoot, A.; Martinez-A, C.; Dorf, M.; Bjerke, T.; Coyle, A.J. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 1998, 188, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Hwang, J.M.; Kubes, P. Modulating leukocyte recruitment in inflammation. J. Allergy Clin. Immunol. Pract. 2007, 120, 3–10. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Yao, X.S.; Li, H.Y.; Dai, Y.; Duan, Y.H.; Li, Y.F.; Kurihara, H. The anti-stress effects of Sarcandra glabra extract on restraint-evoked immunocompromise. Biol. Pharm. Bull. 2009, 32, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Ohigashi, H. Superoxide scavenging activity of rosmarinic acid from Perilla frutescens Britton var. acuta f. viridis. J. Agric. Food Chem. 1998, 46, 4545–4550. [Google Scholar] [CrossRef]
- Shuzhen, C.; Yangping, F.; Ruoshu, W. Effects of rosmarinic acid on free radical production and lysosomal enzyme release from rat peritoneal neutrophils. Acta Pharm. Sin. 1999, 12, 881–885. [Google Scholar]
- Liang, Z.; Nie, H.; Xu, Y.; Peng, J.; Zeng, Y.; Wei, Y.; Wen, X.; Qiu, J.; Zhong, W.; Deng, X.; He, J. Therapeutic effects of Rosmarinic Acid on airway responses in a murine model of asthma. Int. Immunopharmacol. 2016. submitted. [Google Scholar]
- Chu, X.; Wei, M.; Yang, X.; Cao, Q.; Xie, X.; Guan, M.; Wang, D.; Deng, X. Effects of an anthraquinone derivative from Rheum officinale Baill, emodin, on airway responses in a murine model of asthma. Food Chem. Toxicol. 2012, 50, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Ci, X.; He, J.; Wei, M.; Yang, X.; Cao, Q.; Li, H.; Guan, S.; Deng, Y.; Pang, D. A novel anti-inflammatory role for ginkgolide B in asthma via inhibition of the ERK/MAPK signaling pathway. Molecules 2011, 16, 7634–7648. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Guan, M.; Xie, X.; Yang, X.; Xiang, H.; Li, H.; Zou, L.; Wei, J.; Wang, D.; Deng, X. Geniposide inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. Int. Immunopharmacol. 2013, 17, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chu, X.; Guan, M.; Yang, X.; Xie, X.; Liu, F.; Chen, C.; Deng, X. Protocatechuic acid suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2013, 15, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Lim, S.; Liao, W.; Lin, Y.; Thiemermann, C.; Leung, B.P.; Wong, W.F. Glycogen synthase kinase-3β inhibition attenuates asthma in mice. Am. J. Respir. Crit. Care Med. 2007, 176, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.










| Target | Forward | Reverse |
|---|---|---|
| Ym2 | TCCACTTTGAACCACATTCCAAGGC | CGAGAGACTGAGACAGTTCAGGGA |
| AMCase | TGGACACACCTTCATCCTGA | CCTCAGTGGCTCCACTTCTC |
| CCL11 | AAACCATAAACAACCTCCTC | CAATAATCCCACATCTCCTT |
| CCR3 | TCTGCTGAGATGTCCCAATA | TCACCAACAAAGGCGTAG |
| E-selectin | CCCTTCCACAGAACCTACCA | TCAGCAGACATTGCTTCACC |
| β-actin | CTGTCCCTGTATGCCTCTG | ATGTCACGCACGATTTCC |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Xu, Y.; Wen, X.; Nie, H.; Hu, T.; Yang, X.; Chu, X.; Yang, J.; Deng, X.; He, J. Rosmarinic Acid Attenuates Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma. Molecules 2016, 21, 769. https://doi.org/10.3390/molecules21060769
Liang Z, Xu Y, Wen X, Nie H, Hu T, Yang X, Chu X, Yang J, Deng X, He J. Rosmarinic Acid Attenuates Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma. Molecules. 2016; 21(6):769. https://doi.org/10.3390/molecules21060769
Chicago/Turabian StyleLiang, Zhengmin, Yangfeng Xu, Xuemei Wen, Haiying Nie, Tingjun Hu, Xiaofeng Yang, Xiao Chu, Jian Yang, Xuming Deng, and Jiakang He. 2016. "Rosmarinic Acid Attenuates Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma" Molecules 21, no. 6: 769. https://doi.org/10.3390/molecules21060769





