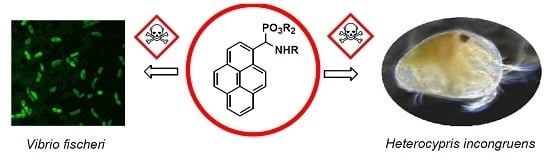
Synthesis, Spectral Characterization of Several Novel Pyrene-Derived Aminophosphonates and Their Ecotoxicological Evaluation Using Heterocypris incongruens and Vibrio fisheri Tests
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Aminophosphonic Derivatives 3a–e, 4 and 5 Bearing 1-Pyrene Moieties
2.2. Ecotoxicological Properties of Aminophosphonates 3a–d and 4
2.2.1. Microtox Toxicity Assay
2.2.2. Ostracod Test Kit
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for Synthesis of Phosphonates 3a–d, 4 and 5
3.1.2. Dimethyl N-(R)-α-Methylbenzylamino(pyren-1-yl)methylphosphonate (4)
3.1.3. Dibenzyl N-(R)-α-Methylbenzylamino(pyren-1-yl)methylphosphonate (5)
3.1.4. Diphenyl N-(R)-α-Methylbenzylamino(pyren-1-yl)methylphosphonate (3e)
3.2. Toxicity Tests
3.2.1. Microtox® Toxicity Assay
3.2.2. Ostracod Test Kit
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kafarski, P.; Lejczak, B.; Tyka, R.; Koba, L.; Pliszczak, E.; Wieczorek, P. Herbicidal activity of phosphonic, phosphinic, and phosphonous acid analogues of phenylglycine and phenylalanine. J. Plant Growth Regul. 1995, 14, 199–203. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Biological Activity of Aminophosphonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic Acids of Potential Medical Importance. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hudson, H.R. Aminophosphonic and Aminophosphinic Acids and their Derivatives as Agrochemicals. In Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Kukhar, V.P., Hudson, H.R., Eds.; Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Maier, L.; Diel, P.J. Organic Phosphorus Compounds 94. Preparation, Physical and Biological Properties of Aminoarylmethylphosphonic- and -Phosphonous Acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 57, 57–64. [Google Scholar] [CrossRef]
- Hudson, H.R. Phosphorus-Containing Fungicides: A Review of Current Research and Prospects. Phosphorus Sulfur Silicon Relat. Elem. 1999, 144–146, 441–444. [Google Scholar] [CrossRef]
- Jane, D.E. Neuroactive aminophosphonic and aminophosphinic acid derivatives. In Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Kukhar, V.P., Hudson, H.R., Eds.; Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Mohd-Pahmi, S.H.; Hussein, W.M.; Schenk, G.; McGeary, R.P. Synthesis, modelling and kinetic assays of potent inhibitors of purple acid phosphatase. Bioorg. Med. Chem. Lett. 2011, 21, 3092–3094. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, A.A.; Kuropatwa, A.; Lewkowski, J.; Szemraj, J. Synthesis of new N-arylamino(2-furyl)methylphosphonic acid diesters, and in vitro evaluation of their cytotoxicity against esophageal cancer cells. Med. Chem. Res. 2013, 22, 852–860. [Google Scholar] [CrossRef]
- Boduszek, B. The Acidic Cleavage of Pyridylmethyl(amino)phosphonates. Formation of the Corresponding Amines. Tetrahedron 1996, 52, 12483–12494. [Google Scholar] [CrossRef]
- Lewkowski, J.; Rzeźniczak, M.; Skowroński, R.; Zakrzewski, J. The first synthesis of ferrocenyl aminophosphonic esters. J. Organomet. Chem. 2001, 631, 105–109. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.E.; Abdel-Kariem, S.M. Methods for the synthesis of α-heterocyclic/heteroaryl-α-aminophosphonic acids and their esters. ARKIVOC 2015, 6, 246–287. [Google Scholar] [CrossRef]
- Kafarski, P.; Górny vel Górniak, M.; Andrasiak, I. Kabachnik-Fields Reaction under Green Conditions—A Critical Overview. Curr. Green Chem. 2015, 2, 218–222. [Google Scholar] [CrossRef]
- Hudson, H.R.; Lee, R.J.; Matthews, R.W. 1-Amino-1-aryl- and 1-Amino-1-heteroaryl-methanephosphonicacids and Their N-Benzhydryl–Protected Diethyl Esters: Preparation and Characterization. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 1691–1709. [Google Scholar] [CrossRef]
- Jayaprakash, S.H.; Uma Maheswara Rao, K.; Satheesh Krishna, B.; Siva Prasad, S.; Syama Sundar, C.; Suresh Reddy, C. PAA-SIO2 Catalyzed Synthesis, Uv Absorption, and Fluorescence Emission Studies of Diethyl (aryl/hetero aryl amino)(pyren-1-yl)-methylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 449–460. [Google Scholar] [CrossRef]
- Lewkowski, J.; Rodriguez Moya, M.; Wrona-Piotrowicz, A.; Zakrzewski, J.; Kontek, R.; Gajek, G. Synthesis, fluorescent properties and the promising cytotoxicity of pyrene-derived aminophosphonates. Beilstein J. Org. Chem. 2016, 12, 1229–1235. [Google Scholar] [CrossRef]
- Ma, X.Y.; Wang, X.C.; Ngo, H.H.; Guo, W.; Wu, M.N.; Wang, N. Bioassay based luminescent bacteria: Interferences, improvements, and applications. Sci. Total. Environ. 2014, 468–469, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Connon, R.E.; Geist, J.; Werner, I. Effect-Based Tools for Monitoring and Predicting the Ecotoxicological Effects of Chemicals in the Aquatic Environment. Sensors 2012, 12, 12741–12771. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rogacz, D.; Rychter, P. The Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants: A Seedling Emergence and Growth Test. Molecules 2016, 21, 694. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, A.; Lewkowski, J.; Rychter, P.; Biczak, R. Phytotoxicity of New Furan-derived Aminophosphonic Acids, N−Aryl Furaldimines and 5−Nitrofuraldimine. J. Agric. Food Chem. 2013, 61, 7673–7678. [Google Scholar] [CrossRef] [PubMed]
- MicroBioTests. Toxkit Advantages/Assets. Available online: http://www.microbiotests.be/information/toxkit-advantagesassets/ (accessed on 19 July 2016).
- Baran, A.; Tarnawski, M. Phytotoxkit/Phytotestkit and Microtoxs as tools for toxicity assessment of sediments. Ecotox. Environ. Safe. 2013, 98, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Weltens, R.; Deprez, K.; Michiels, L. Validation of Microtox as a first screening tool for waste classification. Waste Manage. 2014, 34, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zuo, J.; Tang, X.; Li, R.; Li, Z.; Zhang, F. Toxicity evaluation of pharmaceutical wastewaters using the alga Scenedesmus obliquus and the bacterium Vibrio fischeri. J. Hazard. Mat. 2014, 266, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rosado, D.; Usero, J.; Morillo, J. Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 2016, 153, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Montalban, M.G.; Hidalgo, J.M.; Collado-Gonzalez, M.; Banos, F.G.D.; Víllora, G. Assessing chemical toxicity of ionic liquids on Vibrio fischeri: Correlation with structure and composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Bonnemoy, F.; Charvy, J.-C.; Bohatier, J.; Mallet, C. Toxicity assessment of the maize herbicides S-metolachlor, benoxacor, mesotrione and nicosulfuron, and their corresponding commercial formulations, alone and in mixtures, using the Microtox test. Chemosphere 2013, 93, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, R.; Ceretti, E.; Zerbini, I.; Casale, R.; Gozio, E.; Bertanza, G.; Gelatti, U.; Donato, F.; Feretti, D. Biodegradability, toxicity and mutagenicity of detergents: Integrated experimental evaluations. Ecotoxicol. Environ. Saf. 2012, 84, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.C.; Pereira, J.L.; Cachada, A.; Duarte, A.C.; Goncalves, P.; Sousa, J.P.; Pereira, R. Structural effects of the bioavailable fraction of pesticides in soil: Suitability of elutriate testing. J. Hazard. Mater. 2010, 184, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Burga Perez, K.F.; Charlatchka, R.; Sahli, L.; Ferard, J.-F. New methodological improvements in the Microtox® solid phase assay. Chemosphere 2012, 86, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Stronkhorst, J.; Ciarelli, S.; Schipper, C.A.; Postma, J.F.; Dubbeldam, M.; Vangheluwe, M.; Brils, J.M.; Hooftman, R. Inter-laboratory comparison of five marine bioassays for evaluating the toxicity of dredged material. Aquat. Ecosyst. Health Manag. 2004, 7, 147–159. [Google Scholar] [CrossRef]
- Doe, K.; Scroggins, R.; Mcleay, D.; Wohlgeschaffen, G. Solid-phase test for sediment toxicity using the luminescent bacterium Vibrio fischeri. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.F., Eds.; Springer: Dordrecht, Holland, 2005; Volume 1, pp. 107–136. [Google Scholar]
- ISO. Water Quality—Determination of Fresh Water Sediment Toxicity to Heterocyprisincongruens (Crustacea, Ostracoda), 1st ed.; ISO 14371:2012; The International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; García-Lorenzo, M.L.; Martínez-López, S.; Bech, J.; García-Tenorio, R.; Bolívar, J.P. Use of bioassays for the assessment of areas affected by phosphate industry wastes. J. Geochem. Explor. 2014, 147, 130–138. [Google Scholar] [CrossRef]
- Gouider, M.; Feki, M.; Sayadi, S. Bioassay and use in irrigation of untreated and treated wastewaters from phosphate fertilizer industry. Ecotoxicol. Environ. Saf. 2010, 73, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Cvancarova, M.; Kresinova, Z.; Cajthaml, T. Influence of the bioaccessible fraction of polycyclic aromatic hydrocarbons on the ecotoxicity of historically contaminated soils. J. Hazard. Mater. 2013, 254–255, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Hollert, H. Comparison of sewage sludge toxicity to plants and invertebrates in three different soils. Chemosphere 2011, 83, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.; Nałęcz-Jawecki, G.; Ulfig, K.; Brigmon, R.L. The application of bioassays as indicators of petroleum-contaminated soil remediation. Chemosphere 2005, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2a–d, 4 and 5 are available from the authors.




| Compounds | EC50 (Lower Limit; Upper Limit) | Coefficient of Determination (R2) |
|---|---|---|
| 3a | 495.9 (203.4; 1209) | 0.7632 |
| 3b | 92.63 (65.27; 131.4) | 0.8912 |
| 3c | 214.5 (165.0; 279.0) | 0.9452 |
| 3d | 102.7 (81.83; 128.9) | 0.9571 |
| 4 | 533.1 (439.4; 646.8) | 0.9516 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewkowski, J.; Rodriguez Moya, M.; Chmielak, M.; Rogacz, D.; Lewicka, K.; Rychter, P. Synthesis, Spectral Characterization of Several Novel Pyrene-Derived Aminophosphonates and Their Ecotoxicological Evaluation Using Heterocypris incongruens and Vibrio fisheri Tests. Molecules 2016, 21, 936. https://doi.org/10.3390/molecules21070936
Lewkowski J, Rodriguez Moya M, Chmielak M, Rogacz D, Lewicka K, Rychter P. Synthesis, Spectral Characterization of Several Novel Pyrene-Derived Aminophosphonates and Their Ecotoxicological Evaluation Using Heterocypris incongruens and Vibrio fisheri Tests. Molecules. 2016; 21(7):936. https://doi.org/10.3390/molecules21070936
Chicago/Turabian StyleLewkowski, Jarosław, Maria Rodriguez Moya, Marta Chmielak, Diana Rogacz, Kamila Lewicka, and Piotr Rychter. 2016. "Synthesis, Spectral Characterization of Several Novel Pyrene-Derived Aminophosphonates and Their Ecotoxicological Evaluation Using Heterocypris incongruens and Vibrio fisheri Tests" Molecules 21, no. 7: 936. https://doi.org/10.3390/molecules21070936