Variations in Essential Oil Yield, Composition, and Antioxidant Activity of Different Plant Organs from Blumea balsamifera (L.) DC. at Different Growth Times
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
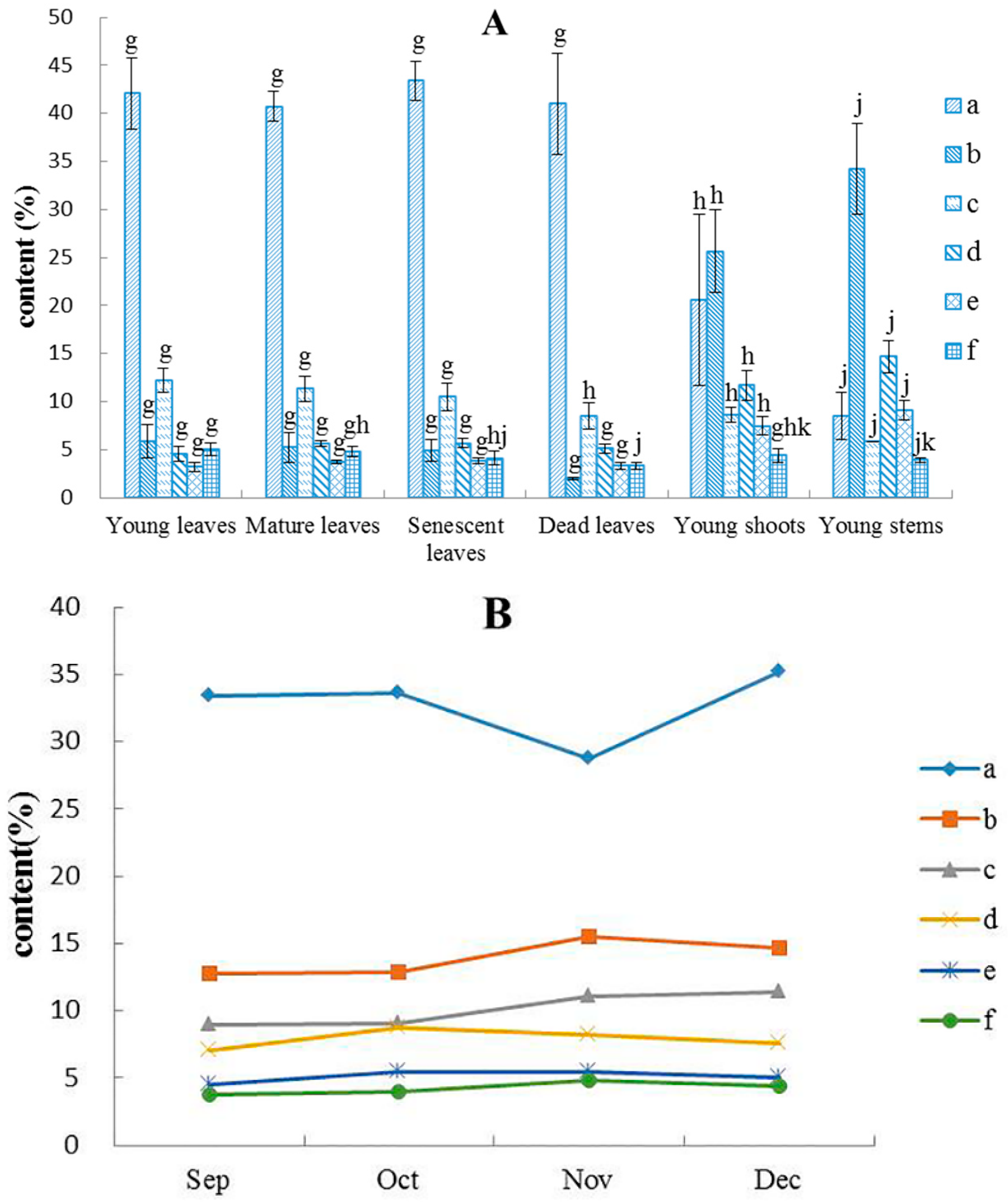
2.1.1. Yields of Essential Oil
2.1.2. Chemical Compositions in Essential Oil
2.1.3. Antioxidant Activity
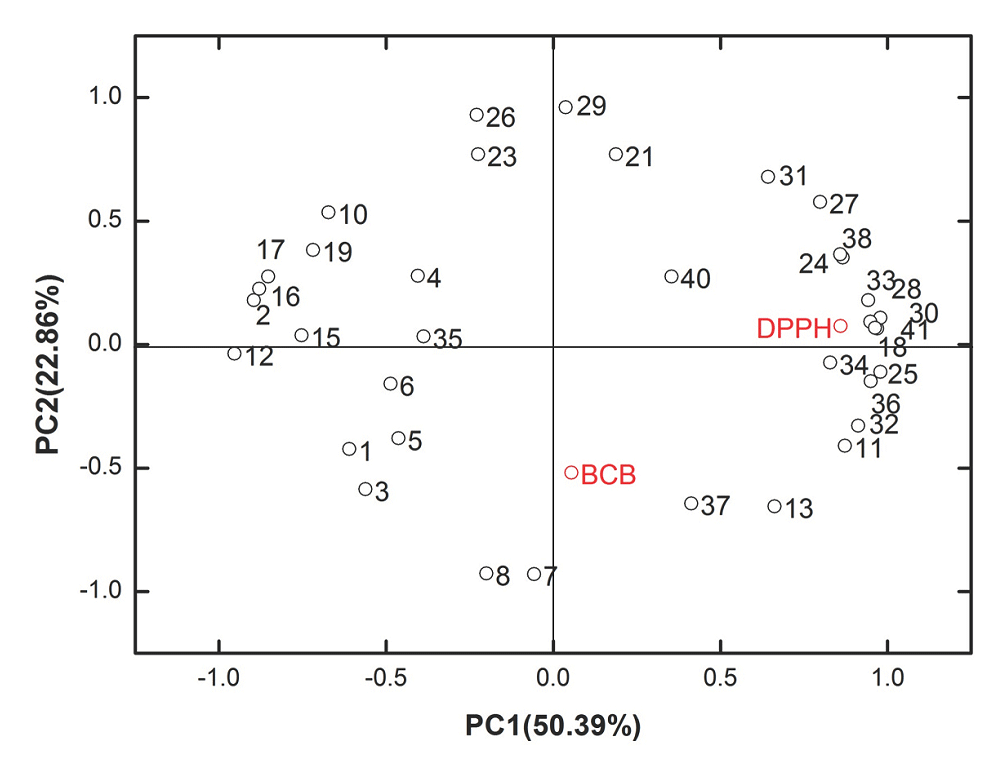
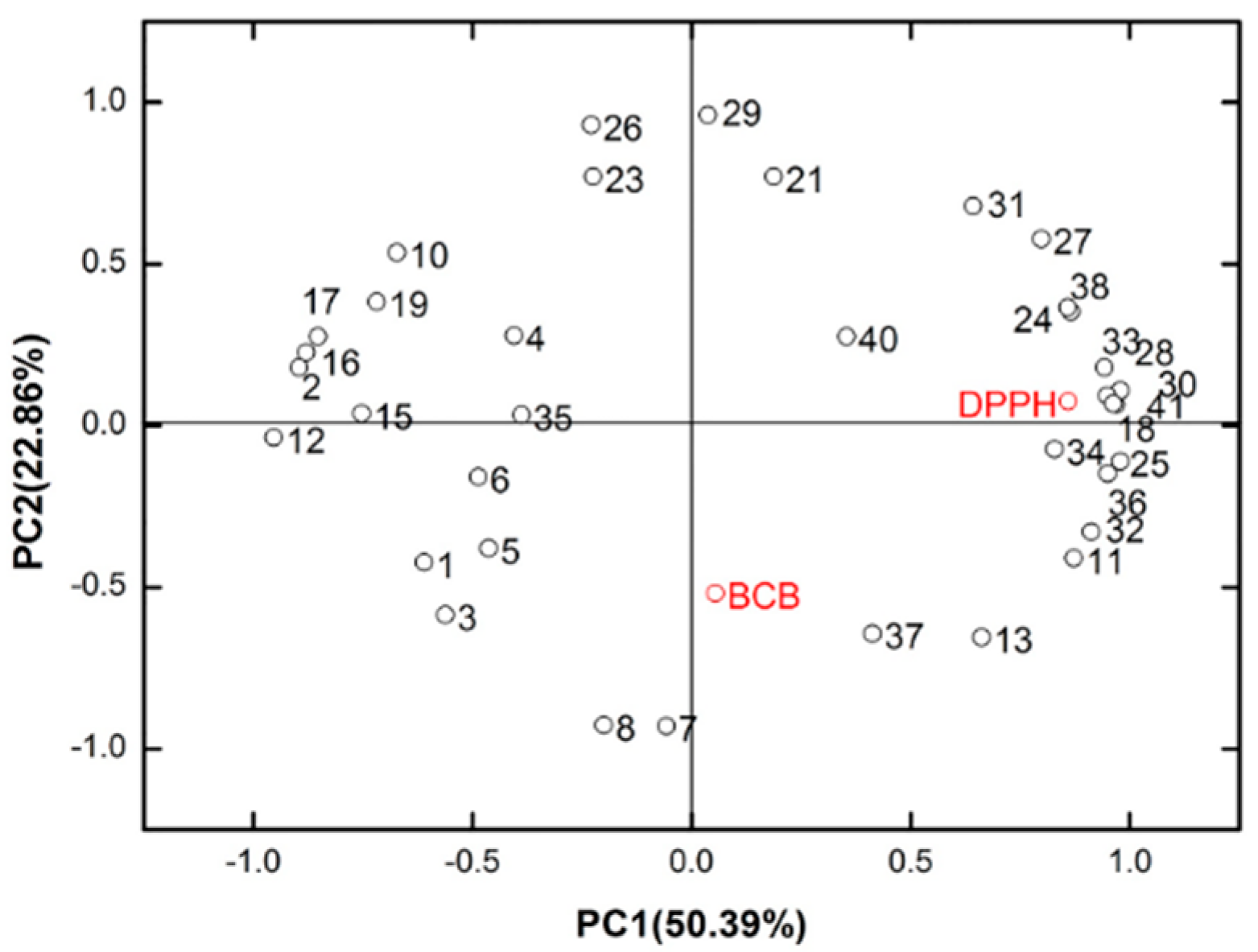
2.1.4. Overall Impact of Plant Organ and Growth Time on the Essential Oils of B. balsamifera
2.2. Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Extraction of Essential Oil
3.4. Analysis of Essential Oil
3.5. Evaluation of Antioxidant Activity
3.5.1. DPPH Radical-Scavenging Assay
3.5.2. β-Carotene Bleaching Test
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pang, Y.X.; Wang, D.; Fan, Z.W.; Chen, X.L.; Yu, F.L.; Hu, X.; Wang, K.; Yuan, L. Blumea balsamifera-A phytochemical and pharmacological review. Molecules 2014, 19, 9453–9477. [Google Scholar]
- Yuan, Y.; Pang, Y.X.; Wang, W.Q.; Zhang, Y.B.; Yu, J.B. Investigation on the plants resources of Blumea balsamifera (L.) DC. in China. J. Trop. Org. 2011, 2, 78–82. [Google Scholar]
- Chinese Academy of Sciences Editorial Commission of the Flora. Flora Republicae Popularis Sinicae; Sciences Press: Beijing, China, 1988; pp. 19–20. [Google Scholar]
- Chen, M.; Qin, J.J.; Fu, J.J.; Hu, X.J.; Liu, X.H.; Zhang, W.D.; Jin, H.Z. Blumeaenes A–J, Sesquiterpenoid esters from Blumea balsamifera with NO inhibitory activity. J. Planta Med. 2010, 76, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Ongsakul, M.; Jindarat, A.; Rojanaworarit, C. Antibacterial effect of crude alcoholic and aqueous extracts of six medicinal plants against Staphylococcus aureus and Escherichia coli. J. Health Res. 2009, 23, 153–156. [Google Scholar]
- Sakee, U.; Maneerat, S.; Cushnie, T.P.; De-Eknamkul, W. Antimicrobial activity of Blumea balsamifera (Lin.) DC. extracts and essential oil. J. Nat. Prod. Res. 2011, 25, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Wang, D.; Pang, Y.X.; Wang, H.; Wang, Z.; Nie, H.; Yu, F.L.; Zhang, Y.B. Effect of Blumea balsamifera oil on percutaneous absorption of salbutamol sulfate. Chin. J. Exp. Tradit. Med. Form. 2013, 19, 174–177. [Google Scholar]
- Wang, D.; Fu, W.J.; Pang, Y.X.; Wang, H.; Hu, X.; Nie, H. The study of skin allergy and acute toxicity of Blumea balsamifera oil. Chin. J. Trop. Crop. 2013, 34, 2499–2502. [Google Scholar]
- Pang, Y.X.; Fan, Z.W.; Wang, D.; Yang, Q.; Wang, K.; Chen, X.L.; Hu, X.; Yu, F.L.; Chen, Z.X. External application of the volatile oil from Blumea balsamifera may be safe for liver-A study on its chemical composition and hepatotoxicity. Molecules 2014, 19, 18479–18492. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.L.; Shi, H.Y.; Qin, J.Z.; Wang, T.W.; Wang, Y.P. Guizhou Miao medicine Blumea balsamifera (L.) DC. and its products. J. Guiyang Coll. Tradit. Chin. Med. 2003, 63–65. [Google Scholar]
- Yu, D.Q. Miao Medicine Industry in Guizhou. Master’s Degree, Central China Normal University, Wuhan, China, 2013. [Google Scholar]
- Guan, L.L.; Pang, Y.X.; Wang, D.; Zhang, Y.B.; Wu, K.Y. Research progress on Chinese Minority Medicine of Blumea balsamifera L. DC. J. Plant Genet. Res. 2012, 13, 695–698. [Google Scholar]
- Bhuiyan, N.I.; Chowdhury, J.U.; Begum, J. Chemical components in volatile oil from Blumea balsamifera (L.) DC. J. Bangladesh J. Bot. 2009, 38, 107–109. [Google Scholar] [CrossRef]
- Chen, M.; Jin, H.Z.; Zhang, W.D.; Yan, S.K.; Shen, Y.H. Chemical constituents of plants from the genus Blumea. J. Chem. Biodivers. 2009, 6, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wang, H.X.; Tian, H.Y.; Ma, C.Y.; Chen, T.; Zou, C.L.; Wang, X. Headspace solid-Phase microextraction coupled with GC-MS for analysis of aromatic components in leaves of Blumea balsamifera (L.) DC. in different seasons. J. Food Sci. 2012, 13, 166–170. [Google Scholar]
- Xia, J.Z.; Zhao, Z.; An, J.; Deng, Y.H. GC fingerprint of herba Blumea from different habitats. J. Chin. Pharm. Aff. 2011, 25, 1191–1194. [Google Scholar]
- Luo, F.L.; Wang, Z.; Zhang, Y.L.; Zhao, Z. Study on medicinal quality of different population and different part of miao medicine Blumea balsamifera DC. J. China Mod. Med. 2013, 20, 51–53. [Google Scholar]
- He, Y.N.; Chai, L.; Ding, Y.; Xian, F.R.; Pan, J.H. Effect of N nutrition on yield and active ingredient in Blumea balsamifera. Guizhou Agric. Sci. 2006, 34, 28–30. [Google Scholar]
- Du, P.; Zhang, X.J.; Sun, X.D. Chemical constituents of volatile oil from Blumea balsamifera (Linn.) DC. in Yunnan. J. Chem. Ind. For. Prod. 2009, 29, 115–118. [Google Scholar]
- Chu, S.S.; Du, S.S.; Liu, Z.L. Fumigant compounds from the essential oil of Chinese Blumea balsamifera leaves against the maize weevil (Sitophilus zeamais). J. Chem. 2013, 2013, 1–7. [Google Scholar]
- Wang, Y.H.; Tian, H.Y.; He, S.J.; Hu, Q.P.; Wang, H.X.; Zou, C.L.; Wang, X. Analysis of volatile components from Blumea balsamifera (L.) DC. leaf with different extraction methods by gas chromatography-mass spectrometry. Sci. Tech. Food Ind. 2012, 12, 97–101, 105. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 2002, 26, 1199–1200. [Google Scholar] [CrossRef]
- Mallet, J.F.; Cerati, C.; Ucciani, E.; Gamisana, J.; Gruber, M. Antioxidant activity of fresh pepper (Capsicum annuum) cultivares. J. Food Chem. 1994, 49, 61–65. [Google Scholar] [CrossRef]
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China, 2010th ed.; The Medicine Science and Technology Press of China: Beijing, China, 2010; p. Appendix 63. [Google Scholar]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidant. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds β-caryophyllene, l-borneol and camphor are available from the authors. Samples of the oils are not available.
| Sample | Oil Amount (mL/100 g, Dry Basis) | ||||
|---|---|---|---|---|---|
| September | October | November | December | Total Mean | |
| Young leaves | 0.75 ± 0.05 a/fg | 0.80 ± 0.10 a/f | 0.61 ± 0.05 a/g | 0.43 ± 0.03 a/h | 0.65 ± 0.16 a |
| Mature leaves | 0.50 ± 0.05 b/f | 0.73 ± 0.07 a/g | 0.49 ± 0.06 b/f | 0.56 ± 0.05 b/f | 0.57 ± 0.11 a |
| Senescent leaves | 0.38 ± 0.06 c/f | 0.56 ± 0.12 b/g | 0.48 ± 0.08 b/fg | 0.59 ± 0.04 b/g | 0.50 ± 0.11 b |
| Dead leaves | 0.23 ± 0.01 d/f | 0.28 ± 0.08 cd/f | 0.23 ± 0.01 c/f | 0.39 ± 0.04 a/g | 0.28 ± 0.08 c |
| Young shoots | 0.40 ± 0.05 c/f | 0.30 ± 0.05 c/g | 0.30 ± 0.00 c/g | 0.21 ± 0.04 c/h | 0.30 ± 0.08 c |
| Young stems | 0.14 ± 0.01 e/f | 0.16 ± 0.01 d/f | 0.13 ± 0.01 d/f | 0.14 ± 0.01 d/f | 0.14 ± 0.02 d |
| Total mean | 0.40 ± 0.2 f | 0.47 ± 0.26 f | 0.36 ± 0.17 f | 0.39 ± 0.17 f | - |
| NO. | Compound Name | RT/min | Relative Content (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | Young Leaves | Mature Leaves | Senescent Leaves | Dead Leaves | Young Shoots | Young Stems | Sep. | Oct. | Nov. | Dec. | |||
| 1 | 1-Octen-3-ol | 8.17 | 969 | 0.71 | 0.85 | 0.49 | - | - | - | 0.77 | 0.83 | 0.34 | 0.53 |
| 2 | Linalool | 11.615 | 1082 | 0.88 | 0.65 | 0.38 | 0.20 | 0.22 | - | 0.54 | 0.53 | 0.54 | 0.46 |
| 3 | Chrysanthenone | 12.39 | 1119 | 0.39 | 0.35 | 0.30 | 0.22 | - | - | 0.42 | 0.32 | 0.28 | 0.27 |
| 4 | 3,3-Dimethyl-6-methylene-cyclohexene | 12.935 | 903 | 0.91 | 0.61 | 0.58 | 0.23 | - | - | 0.65 | 1.27 | 0.50 | 0.60 |
| 5 | Camphor | 12.98 | 1121 | 1.07 | 1.12 | 1.04 | 0.52 | 0.36 | 0.17 | 0.26 | 1.08 | 0.33 | 0.61 |
| 6 | l-Borneol | 13.625 | 1138 | 42.06 | 40.73 | 43.39 | 40.97 | 20.61 | 8.52 | 33.35 | 33.57 | 28.73 | 35.22 |
| 7 | 2,2,8-Trimethyltricyclo[6.2.2.01,6]dodec-5-ene | 18.08 | 1351 | 4.58 | 5.63 | 5.65 | 5.12 | 11.73 | 14.66 | 7.06 | 8.74 | 8.20 | 7.58 |
| 8 | Thujopsene-(I2) | 18.59 | 1512 | 3.18 | 3.71 | 3.81 | 3.34 | 7.47 | 9.06 | 4.51 | 5.45 | 5.41 | 5.01 |
| 9 | (+)-a-Longipinene | 18.772 | 1403 | - | - | - | - | 0.26 | 0.38 | 0.25 | 0.28 | 0.36 | 0.38 |
| 10 | Eugenol | 18.845 | 1392 | 0.23 | 0.21 | - | - | - | - | - | 0.20 | 0.21 | 0.25 |
| 11 | Dehydroaromadendrene | 18.935 | 1396 | - | 0.11 | 0.16 | 0.24 | 0.35 | 1.15 | 0.54 | 0.47 | 0.36 | 0.33 |
| 12 | Thujopsene-I3 | 19.425 | 1416 | 0.18 | 0.16 | 0.12 | 0.09 | 0.74 | 0.94 | 0.40 | 0.46 | 0.47 | 0.33 |
| 13 | Dichloroacetic acid, 2-(1-adamantyl)ethyl ester | 19.61 | 1765 | 0.70 | 0.88 | 0.91 | 0.98 | 2.45 | 3.36 | 1.42 | 1.71 | 1.64 | 1.41 |
| 14 | 2,3,4,5-Tetramethyltricyclo[3.2.1.02,7]oct-3-ene | 19.764 | 1072 | - | - | - | - | 0.27 | 0.38 | 0.29 | 0.37 | 0.35 | 0.30 |
| 15 | Dimethoxydurene | 20.53 | 1511 | 5.85 | 5.23 | 4.95 | 2.01 | 25.64 | 34.20 | 12.78 | 12.86 | 15.52 | 14.68 |
| 16 | β-Caryophyllene | 20.62 | 1494 | 12.24 | 11.36 | 10.52 | 8.51 | 8.62 | 5.85 | 8.90 | 9.04 | 11.08 | 11.34 |
| 17 | α-Caryophyllene | 21.45 | 1579 | 5.02 | 4.77 | 4.11 | 3.29 | 4.37 | 3.92 | 3.81 | 3.98 | 4.78 | 4.43 |
| 18 | (+)-Aromadendrene | 21.635 | 1386 | 0.71 | 0.73 | 0.81 | 1.39 | 0.24 | 0.07 | 0.81 | 0.73 | 0.67 | 0.61 |
| 19 | 4-Methoxy-3-tert-butylphenol | 22.255 | 1417 | 0.53 | 0.37 | 0.29 | 0.26 | 0.37 | - | 0.30 | 0.38 | 0.39 | 0.44 |
| 20 | [(1S,2S,4R)-1,3,3-Trimethyl-norbornan-2-yl] acetate | 23.056 | 1277 | - | - | - | - | - | 0.34 | 0.39 | 0.31 | 0.35 | 0.32 |
| 21 | δ-Cadinene | 23.11 | 1469 | 0.35 | 0.25 | 0.22 | 0.25 | - | - | 0.36 | 0.23 | 0.26 | 0.22 |
| 22 | (−)-Globulol | 23.132 | 1530 | - | - | - | - | 0.25 | 0.42 | 0.56 | 0.33 | 0.38 | 0.22 |
| 23 | Elemol | 23.72 | 1522 | 0.34 | 0.19 | 0.13 | 0.16 | 0.15 | - | 0.19 | 0.19 | 0.20 | 0.19 |
| 24 | (±)−trans-Nerolidol | 23.97 | 1564 | 0.48 | 0.38 | 0.38 | 0.61 | 0.25 | - | 0.52 | 0.37 | 0.45 | 0.37 |
| 25 | Caryophyllene oxide | 24.61 | 1507 | 1.66 | 2.45 | 3.10 | 5.69 | 1.41 | 2.21 | 3.13 | 2.71 | 2.79 | 2.39 |
| 26 | Guaiol | 24.885 | 1614 | 0.76 | 0.53 | 0.39 | 0.54 | 0.43 | - | 0.53 | 0.48 | 0.45 | 0.66 |
| 27 | 1-(1-Oxobutyl)-1,2-dihydropyridine | 24.965 | 1231 | 0.68 | 0.55 | 0.52 | 0.85 | 0.30 | - | 0.65 | 0.50 | 0.61 | 0.55 |
| 28 | Ledol | 25.075 | 1530 | 0.22 | 0.24 | 0.31 | 0.52 | 0.12 | - | 0.36 | 0.26 | 0.28 | 0.25 |
| 29 | γ-Maaliene | 25.455 | 1626 | 0.60 | 0.41 | 0.32 | 0.51 | 0.38 | - | 0.49 | 0.40 | 0.37 | 0.52 |
| 30 | α-Cuparenol | 25.501 | 1776 | - | - | 0.29 | 0.66 | 0.46 | 2.09 | 1.95 | 0.93 | 0.70 | 0.48 |
| 31 | g-Eudesmol | 25.695 | 1626 | 2.34 | 1.84 | 1.79 | 2.41 | 1.70 | 0.40 | 1.92 | 1.77 | 1.96 | 1.80 |
| 32 | Alloaromadendrene oxide-(1) | 25.815 | 1462 | 0.85 | 1.61 | 2.08 | 2.87 | 0.44 | 0.71 | 1.43 | 1.40 | 1.58 | 1.30 |
| 33 | β-Eudesmol | 26.14 | 1593 | 1.63 | 1.66 | 1.66 | 2.91 | 0.92 | 0.27 | 1.79 | 1.48 | 1.49 | 1.28 |
| 34 | Selina-6-en-4-ol | 26.225 | 1593 | 1.59 | 1.52 | 1.65 | 2.65 | - | 0.43 | 1.89 | 1.46 | 1.52 | 1.25 |
| 35 | Xanthoxylin | 26.515 | 1628 | 3.60 | 3.68 | 3.40 | 1.75 | 1.46 | - | 3.91 | 2.92 | 2.46 | 1.90 |
| 36 | 3-Ethyl-3-hydroxy-androstan- 17-one | 26.565 | 2251 | 0.16 | 0.27 | 0.36 | 0.65 | 0.13 | 0.27 | 0.44 | 0.33 | 0.34 | 0.25 |
| 37 | Tetradecanal | 27.34 | 1601 | 0.45 | 0.85 | 1.34 | 1.07 | 0.65 | 2.08 | 0.72 | 0.99 | 1.15 | 1.43 |
| 38 | (−)-Spathulenol | 27.63 | 1536 | 0.09 | - | - | 0.14 | - | - | 0.14 | 0.17 | 0.16 | 0.09 |
| 39 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 31.259 | 4765 | - | - | - | - | - | 4.33 | 3.28 | 4.52 | 5.02 | 4.51 |
| 40 | 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-yl-3-methylenecyclopentane-carboxylate | 31.855 | 1785 | 1.95 | 1.89 | 1.59 | 2.10 | 0.78 | 0.61 | 2.12 | 1.59 | 1.23 | 1.28 |
| 41 | Phytol | 32.995 | 2045 | 0.38 | 0.64 | 0.51 | 1.45 | 0.35 | 0.31 | 0.73 | 0.44 | 0.90 | 0.47 |
| 42 | Linoleic acid | 33.185 | 2183 | - | - | - | - | 0.14 | 0.96 | 1.33 | 0.40 | 0.93 | 0.53 |
| 43 | Tetrahydrofuran-2-carboxylic acid, (9-oxo-9H-fluoren-2-yl) amide | 34.821 | 2707 | - | - | - | - | 1.08 | 1.47 | 0.98 | 1.09 | 1.56 | 1.47 |
| 44 | Cyclopropanecarboxylic acid, 1-methyl-,2,6-bis(1,1-dimethyl-ethyl)-4-methylphenyl ester | 34.931 | 2102 | - | - | - | - | 0.32 | - | 0.40 | 0.35 | 0.23 | - |
| No. | Plant Organ | IC50 Value | |
|---|---|---|---|
| DPPH | BCB | ||
| 1 | Young leaves | 3.36 ± 0.68 a | 1.84 ± 0.34 a |
| 2 | Mature leaves | 5.05 ± 1.86 a,b | 2.44 ± 0.57 b |
| 3 | Senescent leaves | 6.11 ± 2.22 b,d | 2.59 ± 0.68 b |
| 4 | Dead leaves | 14.86 ± 5.92 c | 2.2 ± 0.18 c |
| 5 | Young shoots | 5.75 ± 0.13 a,d | 1.81 ± 0.47 a |
| No. | Month | IC50 Value | |
|---|---|---|---|
| DPPH | BCB | ||
| 1 | September | 9.13 ± 7.31 a | 1.98 ± 0.44 a |
| 2 | October | 7.22 ± 6.04 b | 2.22 ± 0.63 b |
| 3 | November | 7.46 ± 2.53 b | 2.43 ± 0.69 c |
| 4 | December | 4.83 ± 2.99 c | 2.07 ± 0.36 a |
| Soil Characteristics | Experimental Field Characteristics |
|---|---|
| Organic matter (g/kg) | 11.37 |
| pH | 4.94 |
| N (g/kg) | 0.51 |
| P (mg/kg) | 25.33 |
| K (mg/kg) | 33.89 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Huang, M.; Pang, Y.-X.; Yu, F.-L.; Chen, C.; Liu, L.-W.; Chen, Z.-X.; Zhang, Y.-B.; Chen, X.-L.; Hu, X. Variations in Essential Oil Yield, Composition, and Antioxidant Activity of Different Plant Organs from Blumea balsamifera (L.) DC. at Different Growth Times. Molecules 2016, 21, 1024. https://doi.org/10.3390/molecules21081024
Yuan Y, Huang M, Pang Y-X, Yu F-L, Chen C, Liu L-W, Chen Z-X, Zhang Y-B, Chen X-L, Hu X. Variations in Essential Oil Yield, Composition, and Antioxidant Activity of Different Plant Organs from Blumea balsamifera (L.) DC. at Different Growth Times. Molecules. 2016; 21(8):1024. https://doi.org/10.3390/molecules21081024
Chicago/Turabian StyleYuan, Yuan, Mei Huang, Yu-Xin Pang, Fu-Lai Yu, Ce Chen, Li-Wei Liu, Zhen-Xia Chen, Ying-Bo Zhang, Xiao-Lu Chen, and Xuan Hu. 2016. "Variations in Essential Oil Yield, Composition, and Antioxidant Activity of Different Plant Organs from Blumea balsamifera (L.) DC. at Different Growth Times" Molecules 21, no. 8: 1024. https://doi.org/10.3390/molecules21081024