A Systematic Review on the Implication of Minerals in the Onset, Severity and Treatment of Periodontal Disease
Abstract
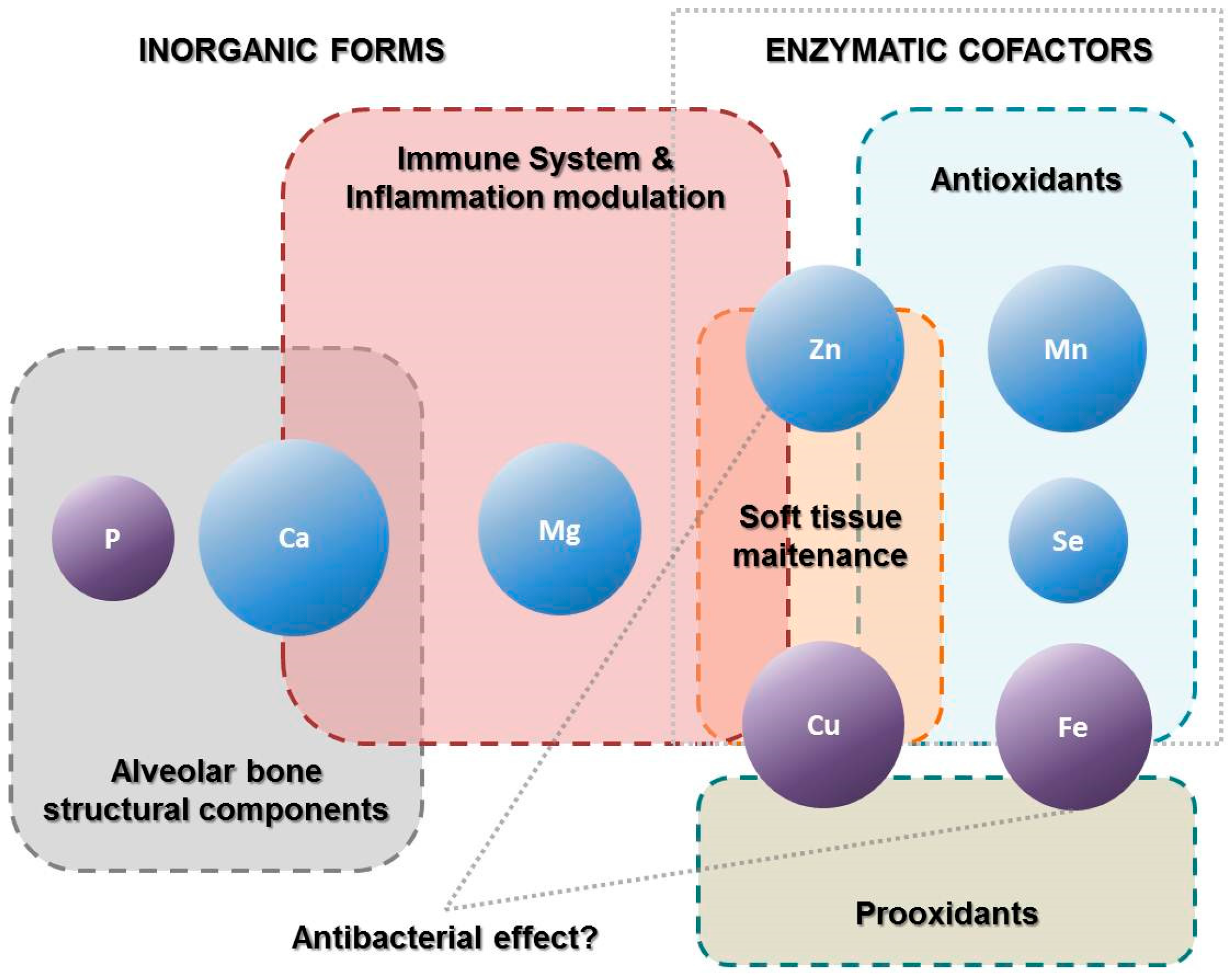
:1. Introduction
2. Results
2.1. Calcium
2.2. Magnesium
2.3. Phosphorus
2.4. Iron
2.5. Copper
2.6. Zinc
2.7. Potassium
2.8. Manganese
2.9. Selenium
3. Discussion
4. Materials and Methods
4.1. Selection Criteria
4.2. Information Source and Search Terms
4.3. Search Strategy
4.4. Data Collection Process, Data Items and Summary Measures
4.5. Quality Assessment and Risk of Bias
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schifferle, R.E. Periodontal disease and nutrition: Separating the evidence from current fads. Periodontol. 2000 2009, 50, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Enwonwu, C.O.; Phillips, R.S.; Ibrahim, C.D.; Danfillo, I.S. Nutrition and oral health in Africa. Int. Dent. J. 2004, 54, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Enwonwu, C.O.; Phillips, R.S.; Falkler, W.A. Nutrition and oral infectious diseases: State of the science. Compend. Contin. Educ. Dent. 1995 2002, 23, 431–448. [Google Scholar]
- Arora, N.; Bansal, M.P.; Koul, A. Azadirachta indica acts as a pro-oxidant and modulates cell cycle associated proteins during DMBA/TPA induced skin carcinogenesis in mice. Cell Biochem. Funct. 2013, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; Hildebolt, C.F.; Miley, D.D.; Garcia, M.N.; Pilgram, T.K.; Couture, R.; Anderson Spearie, C.; Civitelli, R. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br. Dent. J. 2009, 206, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Raindi, D. Nutrition and Periodontal Disease. Dent. Update 2015, 43, 66–68. [Google Scholar]
- Chapple, I.L. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin. Mol. Pathol. 1996, 49, M247–M255. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Oral contraceptives alter oral health. Ann. Saudi Med. 2010, 30, 243. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Bhatavadekar, N.B.; Uttamani, J.R. The effect of nutrition on periodontal disease: A systematic review. J. Calif. Dent. Assoc. 2014, 42, 302–311. [Google Scholar] [PubMed]
- Varela-López, A.; Quiles, J.L.; Cordero, M.; Giampieri, F.; Bullón, P. Oxidative Stress and Dietary Fat Type in Relation to Periodontal Disease. Antioxid. Basel Switz. 2015, 4, 322–344. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, G.-J.; Vanobbergen, J.; De Visschere, L.; Schols, J.; de Baat, C. Association of some specific nutrient deficiencies with periodontal disease in elderly people: A systematic literature review. Nutrtion 2009, 25, 717–722. [Google Scholar]
- Van der Velden, U.; Kuzmanova, D.; Chapple, I.L.C. Micronutritional approaches to periodontal therapy. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 142–158. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Varela-López, A. The Role of Nutrition in Periodontal Diseases. In Studies on Periodontal Disease; Springer: New York, NY, USA, 2014; pp. 251–278. [Google Scholar]
- Schifferle, R.E. Nutrition and periodontal disease. Dent. Clin. N. Am. 2005, 49, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Al-Shammari, K.; Nociti, F.H.; Soehren, S.; Wang, H.-L. Effects of Vitamin-B complex supplementation on periodontal wound healing. J. Periodontol. 2005, 76, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Bullón, P.; Giampieri, F.; Quiles, J.L. Non-Nutrient, Naturally Occurring Phenolic Compounds with Antioxidant Activity for the Prevention and Treatment of Periodontal Diseases. Antioxid. Basel Switz. 2015, 4, 447–481. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Battino, M.; Bullón, P.; Quiles, J.L. Dietary antioxidants for chronic periodontitis prevention and its treatment: A review on current evidences from animal and human studies. Ars Pharm. Internet 2015, 56, 131–140. [Google Scholar] [CrossRef]
- Milward, M.R.; Chapple, I.L.C. The role of diet in periodontal disease. Clin Dent Health 2013, 52, 18–21. [Google Scholar]
- Amaral, C.D.S.F.; Vettore, M.V.; Leão, A. The relationship of alcohol dependence and alcohol consumption with periodontitis: A systematic review. J. Dent. 2009, 37, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Grossi, S.G.; Dunford, R.G.; Ho, A.W.; Trevisan, M.; Genco, R.J. Calcium and the Risk for Periodontal Disease. J. Periodontol. 2000, 71, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, A.R.; Boucher, B.J.; Kongstad, J.; Fiehn, N.-E.; Christensen, L.B.; Heitmann, B.L. Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr. 2016, 19, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Freeland, J.H.; Cousins, R.J.; Schwartz, R. Relationship of mineral status and intake to periodontal disease. Am. J. Clin. Nutr. 1976, 29, 745–749. [Google Scholar] [PubMed]
- Tanaka, K.; Miyake, Y.; Okubo, H.; Hanioka, T.; Sasaki, S.; Miyatake, N.; Arakawa, M. Calcium intake is associated with decreased prevalence of periodontal disease in young Japanese women. Nutr. J. 2014, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Morita, M.; Akhter, R.; Akino, K.; Honda, O. Relationship between folic acid intake and gingival health in non-smoking adults in Japan. Oral Dis. 2010, 16, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, A.R.A.; Christensen, L.B.; Holm-Pedersen, P.; Avlund, K.; Boucher, B.J.; Heitmann, B.L. Intake of dairy products in relation to periodontitis in older Danish adults. Nutrients 2012, 4, 1219–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegboye, A.R.A.; Fiehn, N.-E.; Twetman, S.; Christensen, L.B.; Heitmann, B.L. Low calcium intake is related to increased risk of tooth loss in men. J. Nutr. 2010, 140, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Meng, H.; Tang, X.; Xu, L.; Zhang, L.; Chen, Z.; Shi, D.; Feng, X.; Lu, R. Elevated plasma calcifediol is associated with aggressive periodontitis. J. Periodontol. 2009, 80, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Cairella, G.; Tarsitani, G. Nutritional variables related to gingival health in adolescent girls. Community Dent. Oral Epidemiol. 2000, 28, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Amarasena, N.; Yoshihara, A.; Hirotomi, T.; Takano, N.; Miyazaki, H. Association between serum calcium and periodontal disease progression in non-institutionalized elderly. Gerodontology 2008, 25, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Ohtsuka-Isoya, M.; Shimauchi, H.; Shinoda, H. Effects of lactation on alveolar bone loss in experimental periodontitis. J. Periodontol. 2007, 78, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Ohtsuka-Isoya, M.; Horiuchi, H.; Shinoda, H. Bone mineral density of alveolar bone in rats during pregnancy and lactation. J. Periodontol. 2000, 71, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Teófilo, J.M.; Azevedo, A.C.B.; Petenusci, S.O.; Mazaro, R.; Lamano-Carvalho, T.L. Comparison between two experimental protocols to promote osteoporosis in the maxilla and proximal tibia of female rats. Pesqui. Odontol. Bras. Braz. Oral Res. 2003, 17, 302–306. [Google Scholar] [CrossRef]
- Messer, H.H.; Goebel, N.K.; Wilcox, L. A comparison of bone loss from different skeletal sites during acute calcium deficiency in mice. Arch. Oral Biol. 1981, 26, 1001–1004. [Google Scholar] [CrossRef]
- De Albuquerque Taddei, S.R.; Madeira, M.F.M.; de Abreu Lima, I.L.; Queiroz-Junior, C.M.; Moura, A.P.; Oliveira, D.D.; Andrade, I.; da Glória Souza, D.; Teixeira, M.M.; da Silva, T.A. Effect of Lithothamnium sp and calcium supplements in strain- and infection-induced bone resorption. Angle Orthod. 2014, 84, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Zmysłowska, E.; Ledzion, S.; Jedrzejewski, K. Factors affecting mandibular residual ridge resorption in edentulous patients: A preliminary report. Folia Morphol. 2007, 66, 346–352. [Google Scholar]
- Meisel, P.; Schwahn, C.; Luedemann, J.; John, U.; Kroemer, H.K.; Kocher, T. Magnesium deficiency is associated with periodontal disease. J. Dent. Res. 2005, 84, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Iwasaki, M.; Miyazaki, H. Mineral content of calcium and magnesium in the serum and longitudinal periodontal progression in Japanese elderly smokers. J. Clin. Periodontol. 2011, 38, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Yamori, M.; Njelekela, M.; Mtabaji, J.; Yamori, Y.; Bessho, K. Hypertension, periodontal disease, and potassium intake in nonsmoking, nondrinker african women on no medication. Int. J. Hypertens. 2011, 2011, 695719. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Kitamura, M.; Kawashita, Y.; Ando, Y.; Saito, T. Periodontal disease and percentage of calories from fat using national data. J. Periodontal Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Kumari, S.; Ramitha, K.; Ashwini Kumari, M.B. Comparative evaluation of micronutrient status in the serum of diabetes mellitus patients and healthy individuals with periodontitis. J. Indian Soc. Periodontol. 2010, 14, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Ramesh, A.; Suresh, S.; Prasad, B.R. A comparative evaluation of antioxidant enzymes and selenium in the serum of periodontitis patients with diabetes mellitus type 2. Contemp. Clin. Dent. 2013, 4, 176–180. [Google Scholar] [PubMed]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Strålfors, A.; Thilander, H.; Bergenholtz, A. Simultaneous inhibition of caries and periodontal disease in hamster by disinfection, tooth-brushing or phosphate addition. Arch. Oral Biol. 1967, 12, 1367–1373. [Google Scholar] [CrossRef]
- Lütfioğlu, M.; Sakallioğlu, U.; Sakallioğlu, E.E.; Bariş, S.; Gürgör, P. The impact of dietary induced hyperparathyroidism on healthy and diseased periodontia: An experimental study in rats. J. Clin. Periodontol. 2012, 39, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Lütfioğlu, M.; Sakallioğlu, U.; Sakallioğlu, E.E.; Diraman, E.; Ciftçi, G.; Tutkun, F. Dietary-induced hyperparathyroidism affects serum and gingival proinflammatory cytokine levels in rats. J. Periodontol. 2010, 81, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Uçkardeş, Y.; Ozmert, E.N.; Unal, F.; Yurdakök, K. Effects of zinc supplementation on parent and teacher behaviour rating scores in low socioeconomic level Turkish primary school children. Acta Paediatr. 2009, 98, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Orbak, R.; Kara, C.; Ozbek, E.; Tezel, A.; Demir, T. Effects of zinc deficiency on oral and periodontal diseases in rats. J. Periodontal Res. 2007, 42, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Seyedmajidi, S.A.; Seyedmajidi, M.; Moghadamnia, A.; Khani, Z.; Zahedpasha, S.; Jenabian, N.; Jorsaraei, G.; Halalkhor, S.; Motallebnejad, M. Effect of zinc-deficient diet on oral tissues and periodontal indices in rats. Int. J. Mol. Cell. Med. 2014, 3, 81–87. [Google Scholar] [PubMed]
- Kaye, E.K. Nutrition, dietary guidelines and optimal periodontal health. Periodontol. 2000 2012, 58, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Coulston, A.M.; Boushey, C.J.; Ferruzzi, M. Nutrition in the Prevention and Treatment of Disease, 3rd ed.; Academic Press: San Diego, CA, USA, 2013; p. 903. [Google Scholar]
- Mayne, S.T. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J. Nutr. 2003, 133, 933S–940S. [Google Scholar] [PubMed]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef] [PubMed]
- DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68,500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 2010, 340, b5463. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.M.P.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet Lond. Engl. 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Carreira, L.M.; Dias, D.; Azevedo, P. Relationship between Gender, Age, and Weight and the Serum Ionized Calcium Variations in Dog Periodontal Disease Evolution. Top. Companion Anim. Med. 2015, 30, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Emelyanov, A.; Koh, C.H.V.; Spitsbergen, J.M.; Lam, S.H.; Mathavan, S.; Parinov, S.; Gong, Z. A high level of liver-specific expression of oncogenic KrasV12 drives robust liver tumorigenesis in transgenic zebrafish. Dis. Model Mech. 2011, 4, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, E.R.; Shoemaker, C.J.; Menke, S.M.; Edelmann, R.E.; Actis, L.A. Evaluation of different iron sources and their influence in biofilm formation by the dental pathogen Actinobacillus actinomycetemcomitans. J. Med. Microbiol. 2007, 56, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Francesca, B.; Ajello, M.; Bosso, P.; Morea, C.; Andrea, P.; Giovanni, A.; Piera, V. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals 2004, 17, 271–278. [Google Scholar] [CrossRef]
- Johnson, M.; Cockayne, A.; Morrissey, J.A. Iron-regulated biofilm formation in Staphylococcus aureus Newman requires ica and the secreted protein Emp. Infect. Immun. 2008, 76, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [PubMed]
- International Zinc Nutrition Consultative Group (IZiNCG); Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar] [PubMed]
- Tubek, S. Selected zinc metabolism parameters in premenopausal and postmenopausal women with moderate and severe primary arterial hypertension. Biol. Trace Elem. Res. 2007, 116, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.O.; Zavaleta, N.; Caulfield, L.E.; Wen, J.; Abrams, S.A. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J. Nutr. 2000, 130, 2251–2255. [Google Scholar] [PubMed]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [PubMed]
- Tamura, M.; Ochiai, K. Zinc and copper play a role in coaggregation inhibiting action of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2009, 24, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Almerich, J.M.; Cabedo, B.; Ortolá, J.C.; Poblet, J. Influence of alcohol in mouthwashes containing triclosan and zinc: An experimental gingivitis study. J. Clin. Periodontol. 2005, 32, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Harrap, G.J.; Best, J.S.; Saxton, C.A. Human oral retention of zinc from mouthwashes containing zinc salts and its relevance to dental plaque control. Arch. Oral Biol. 1984, 29, 87–91. [Google Scholar] [CrossRef]
- Addy, M.; Richards, J.; Williams, G. Effects of a zinc citrate mouthwash on dental plaque and salivary bacteria. J. Clin. Periodontol. 1980, 7, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Agabegi, S.S.; Stern, P.J. Bias in research. Am. J. Orthop. 2008, 37, 242–248. [Google Scholar] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. Lond. Engl. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
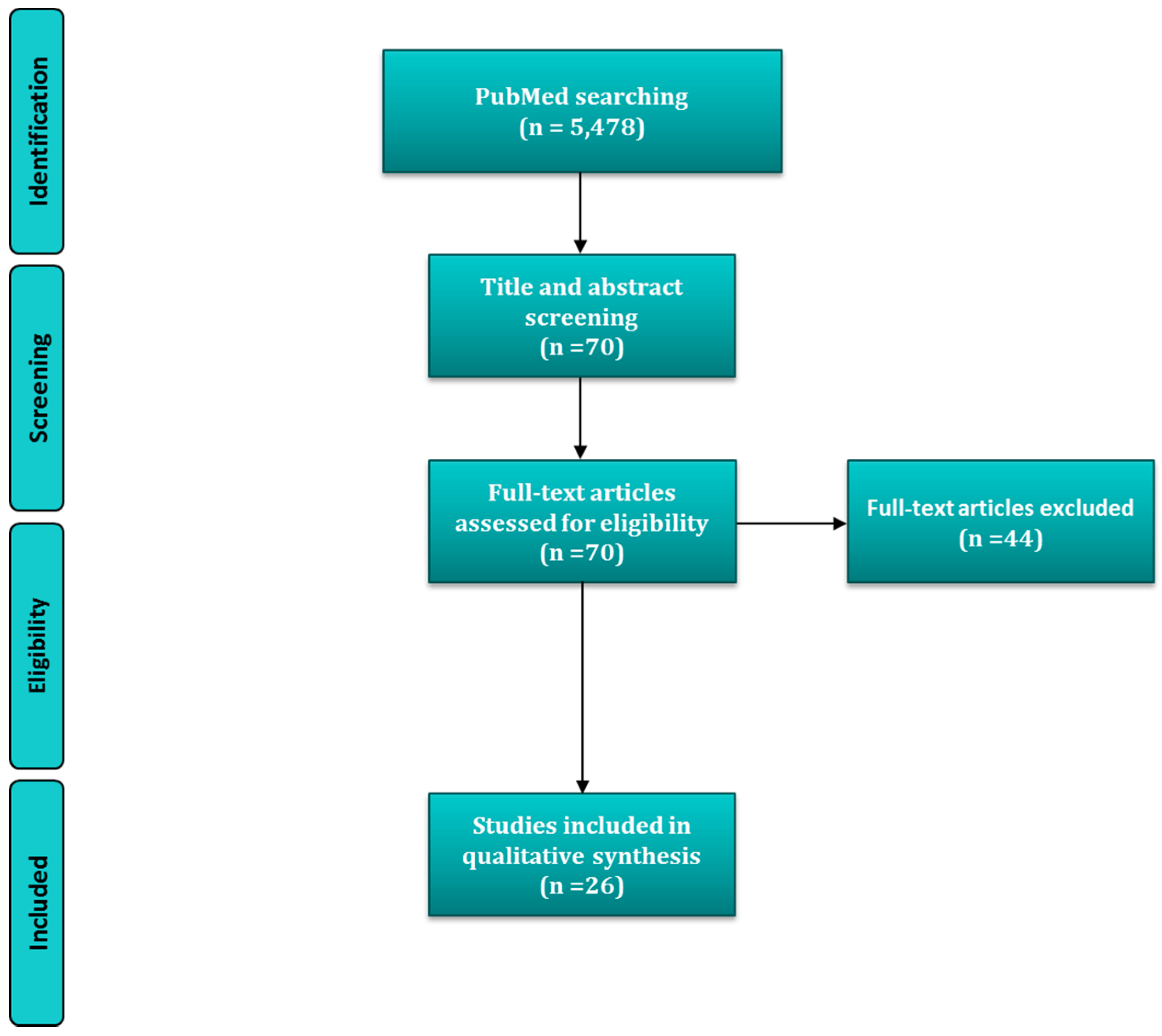
| Study Type | Sample | Sex, Age, N | Dietary Intake Assessment | Nutritional Status Assessment | Periodontal Status | Association Assessment | Main Results/Conclusions | Ref. |
|---|---|---|---|---|---|---|---|---|
| CS | NHANES III participants (USA) | Both, ≥ 20 years, N = 11787 | Dietary intake of Ca (<500, 500–799, & ≥ 800 mg/day) by 24-h recall | Serum levels 1 of total Ca | Periodontal disease (CAL > 1.5 mm) | Adj OR (multiple logistic regression) | Inverse association with intake & serum levels but only in certain age groups & gender | [20] |
| CS | 2007–2008 DANHES participants (Denmark) | Both, ≥18 years, N = 3287 | Dietary intake of Ca (continous, & < & ≥ RDA) by FFQ | - | Severe chronic periodontitis 2 mean CAL | Adj OR (multiple logistic regression) Adj β (multiple linear regression) | Negative association with mean CAL for intakes ≥ RDA | [21] |
| CS | Dental clinic patients (USA) | Both, N/A, N = 80 | Dietary intakes of Ca, P, Fe, Zn & K by 24-h recall (N = 56) | Serum levels of Ca, Mg, P (Fe, Cu, Zn & K) | RPI | Linear correlation coefficient for intake Adj β for serum levels (multiple linear regression) | Inverse association with Cu serum levels | [22] |
| CS | Pregnant women (Japan) | Female, 31.5 ± 4 years, N = 1162 | Dietary intake of Ca (quartiles) by DHQ | - | Periodontal disease (PPD ≥ 4 mm in ≥1 tooth) | Adj OR (multiple logistic regression) | Inverse association (highest qt vs. lowest qt) | [23] |
| CS | Non-smoker adults with ≥20 teeth (Japan) | Both, ≥18 years, N = 497 | Dietary intake of Ca by 24-h recall | - | CPI & %BOP | Adj β (multiple linear regression) | No associations | [24] |
| CS | SHIP participants (Germany) | Both, 20–80 years, N = 4290 | Ca-antagonists regular use & Mg-containing drugs intake | Serum Mg/Ca (quartiles) | % PPD ≥ 4 mm, %CAL > 4 mm, number of teeth | Adj OR (multiple logistic regression) | Inverse association of serum Mg/Ca with %PPD > 4 mm & %CAL > 4 mm, only in subjects aged ≥ 40 years (lowest quartil vs. highest quartil). People taking Mg-containing drugs showed less CAL | [36] |
| S | 135 subjects from 3rd follow-up of COHSS (Denmark) | Both, >65 years, N = 135 | Dietary intakes of dairy food & Ca (100 g increments) by DHI | - | Periodontitis (number of teeth with additional CAL ≥ 3 mm) | Adj IRR (multiple logistic regression) | Inverse association with intakes of total dairy Ca, from milk & fermented foods | [25] |
| CS | non-smokers & non-alcoholic women from CARDIAC study (Tanzania) | Female, 46–58 years, N = 81 | Dietary intake of Mg (N/A) & 24-h urinary excretions of K | - | number of teeth, & CPITN | Simple correlation coefficients | Negative association of K urinary levels with CPITN | [38] |
| CS | NHANES non-pregnant participants (Japan) | Both, ≥20 years, N = 3043 | Dietary intake of Cu by 24-h recall | - | CPI = 3–4 | Adj OR (multiple logistic regression) | No association | [39] |
| CS | KNHANES participants (South korea) | Both, ≥19 years, N = 1679 | - | Whole blood levels of Mn (quartiles) | CPI ≥ 3 | Adj OR (multiple logistic regression) | Inverse asociation | [40] |
| CC | Female non-smoker adolescents (Italy) | Female, 17–19 years, N = 54 | Dietary intake of Ca, P & K (<2/3 RDA) by 3-days record | - | Gingivitis-affected (≥1 site with BOP) vs. non-affected | Differences between groups | Negative association of Ca intake with gingivitis risk | [28] |
| CC | Subjects from a Health Center (China) | Both, 16–64 years, N = 178 | - | Plasma level of Ca & P (quartiles) | Aggressive periodontitis vs. chronic periodontitis—affected 3 vs. healthy (Staff) | Differencies among groups (ANCOVA) | Patints had lower levels of P | [27] |
| CC | Non-smokers outpatients (India) | Both, 30–60 years, N = 60 | - | Serum levels of Cu & Zn | DM2 & periodontal disease-affected vs. only periodontal disease-affected (30% sites with CAL ≥ 5 mm & BOP) vs. healthy | Differences among groups | Subjects with perioedontitis showed higher Zn levels respect to than those with DM2 | [41] |
| CC | Non-smokers individuals with ≥20 teeth (India) | Both, 30–60 years, N = 150 | - | Serum levels of Se | DM2 & chronic periodontitis-affected 4, only chronic periodontitis-affected 4 vs. healthy | Differences between groups | Subjects with periodontitis (with or without DM2) showed the lowest Se levels | [42] |
| C (5 years) | MONICA study participants (Denmark) | Both, 30–60 years, N = 2113 | Dietary intakes of Ca (total, E-adjusted as well as < & ≥ RDA) by 7-days record | - | Tooth loss | Adj IRR (multiple logistic regression) | Inverse association only in men | [26] |
| C (6 years) | Niigata city citizens (Japan) | Both, 70 years, N = 266 | - | Serum levels of Ca | Progression of periodontal diseae (number of teeth with additional CAL ≥ 3 mm) | Adj β (multiple linear regression) | Negative association | [29] |
| C (6 years) | Niigata city inhabitants (Japan) | Both, 73 years, N = 309 | - | Serum Ca/Mg (quartiles) | Periodontal disease events (CAL ≥ 3 mm/year at any teeth) | Adj OR (multiple logistic regression) | Negative association of Ca/Mg ratio with periodontal disease events only among smokers | [37] |
| Subjects/Animals, Age, Sample Size (N) | Experimental Design (Duration) | Main Results/Conclusions | Ref. |
|---|---|---|---|
| Pregnant & non-pregnant Wistar rats, 10 weeks, N = 62 | Diets containing 0.9%, 0.3%, or 0.02% Ca (6 weeks). In pregnant rats, it includes gestation & lactation periods (3 weeks each) | BMD of alveolar bone decreased with Ca intake, but this decrease was greater in pregnant rats. Mother’s intake also affected to Pups‘ BMD | [31] |
| Pregnant (lactating) & non-pregnant (non-lactating) Wistar rats, 10 weeks, N = 62 | Diets containing 0.9%, 0.3%, or 0.02% Ca (46 days) with experimental periodontitis induced by an elastic ring (last 2 weeks). Pregnant rats started lactation on d 25 | BMD & ACH decreased in periodontitis side according to the Ca intake, but this was greater in the lactating group | [30] |
| Male BALB/c mice inoculated with Aact, 18 weeks, N = 40 | Regular or CaCO3-rich diet (30 or 60 days) | Ca-rich diet fed animals showed lower osteoclasts, ABL & levels of TNF-α in periodontal tissues | [34] |
| Syrian hamsters, N/A, N = 10 | Diet with 5% Ca3(PO4)2 or a mixture of Na2HPO4 & KH2PO4 (90 days or 150 days) | Ca3(PO4)2-rich diet inhibited ABL | [44] |
| Male SpDw rats, 3 months, N = 24 | Ca/P imbalanced diet to induced dHPT or standard diet (5 months), following by LPS- or saline-injections (next 2 weeks) | Rats with dHPT & periodontitis revealed the highest amounts of inflammatory cells & vessels as well as of ABL & AL, followed by rats with only periodontitis & with only dHPT. | [45] |
| SpDw rats, Adults, N = 24 | Ca/P imbalanced diet to induced dHPT or standard diet (5 months), following by LPS- or saline-injections (next 2 weeks) | IL-1β & TNF-α were highest in rats with dHPT & periodontis, followed by those with only LPS-treated. They were positively correlated to PTH levels in rats with only dHPT | [46] |
| Children (India), 8.50 ± 0.7 years, N = 68 | Orally-administed elemental Zn supplements or placebo syrup DB (10 weeks) | Zn supplements improved PI, but not GI | [47] |
| SpDw rats, at weaning, N = 14 | Zn-deficient or normal diet (4 weeks) | Rats fed Zn-deficient diet showed higher PI & GI. No differences were observed for PPD score | [48] |
| male Wistar rats, at weaning (24 days), N = 14 | Zn-deficient or Zn-containing diet (4 weeks) | Aphthous ulcer on the floor of the mouth was observed in Zn-deficient rats, which showed higher GI. No differences were observed for PPD score | [49] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-López, A.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. A Systematic Review on the Implication of Minerals in the Onset, Severity and Treatment of Periodontal Disease. Molecules 2016, 21, 1183. https://doi.org/10.3390/molecules21091183
Varela-López A, Giampieri F, Bullón P, Battino M, Quiles JL. A Systematic Review on the Implication of Minerals in the Onset, Severity and Treatment of Periodontal Disease. Molecules. 2016; 21(9):1183. https://doi.org/10.3390/molecules21091183
Chicago/Turabian StyleVarela-López, Alfonso, Francesca Giampieri, Pedro Bullón, Maurizio Battino, and José L. Quiles. 2016. "A Systematic Review on the Implication of Minerals in the Onset, Severity and Treatment of Periodontal Disease" Molecules 21, no. 9: 1183. https://doi.org/10.3390/molecules21091183






