Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of GO-CS-PHGC Composites
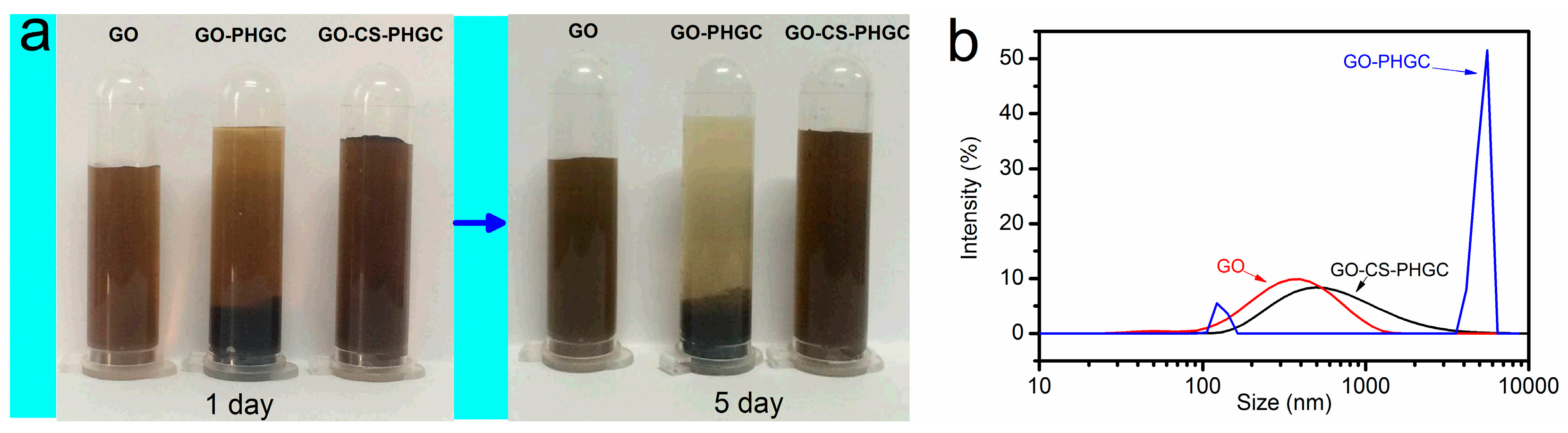
2.1.1. The Stability of GO-CS-PHGC Composites in Aqueous Solution
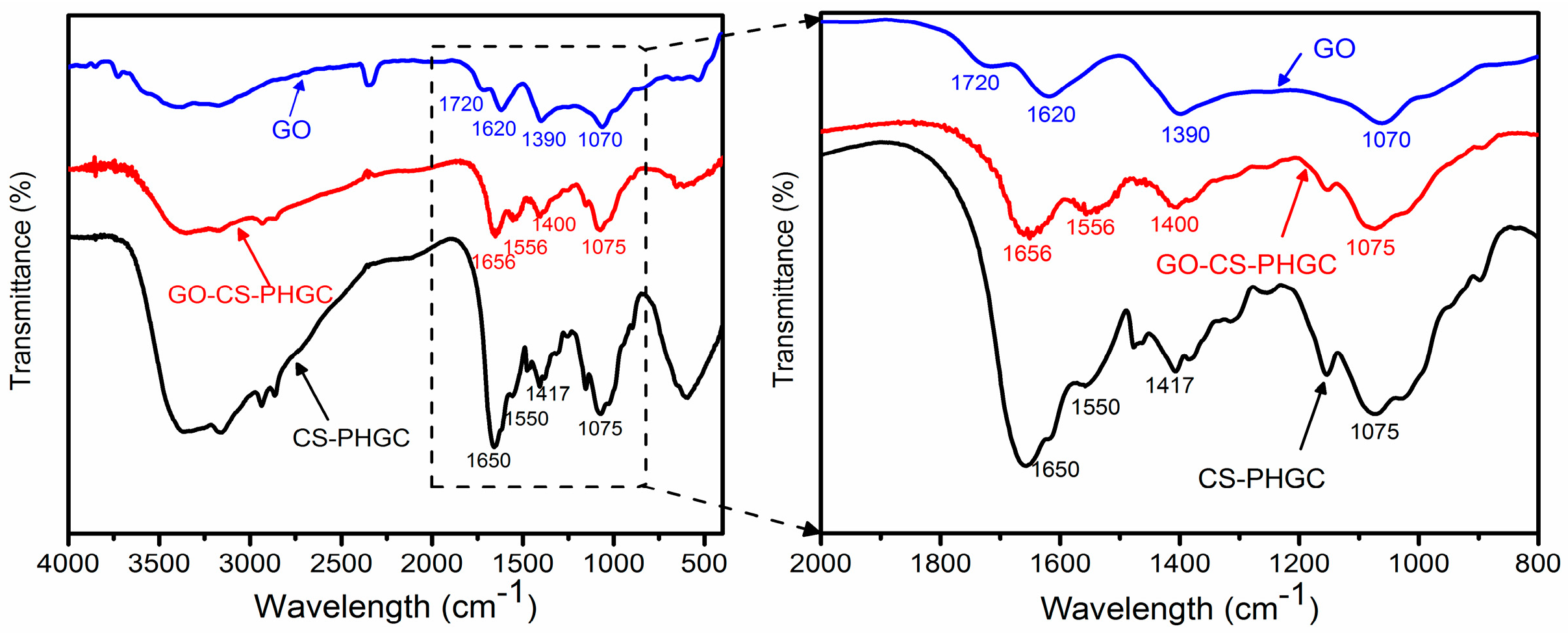
2.1.2. FTIR Analysis
2.1.3. XPS Analysis
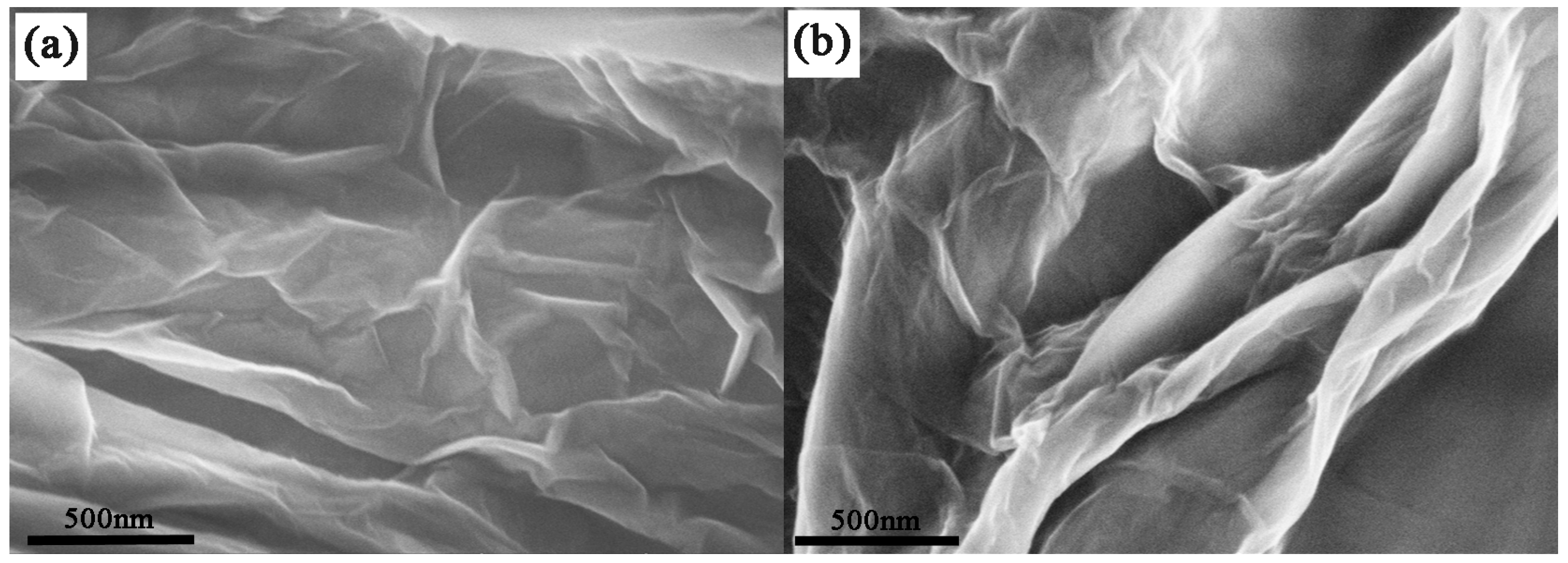
2.1.4. FE-SEM and TEM Analysis
2.1.5. Raman Spectroscopy
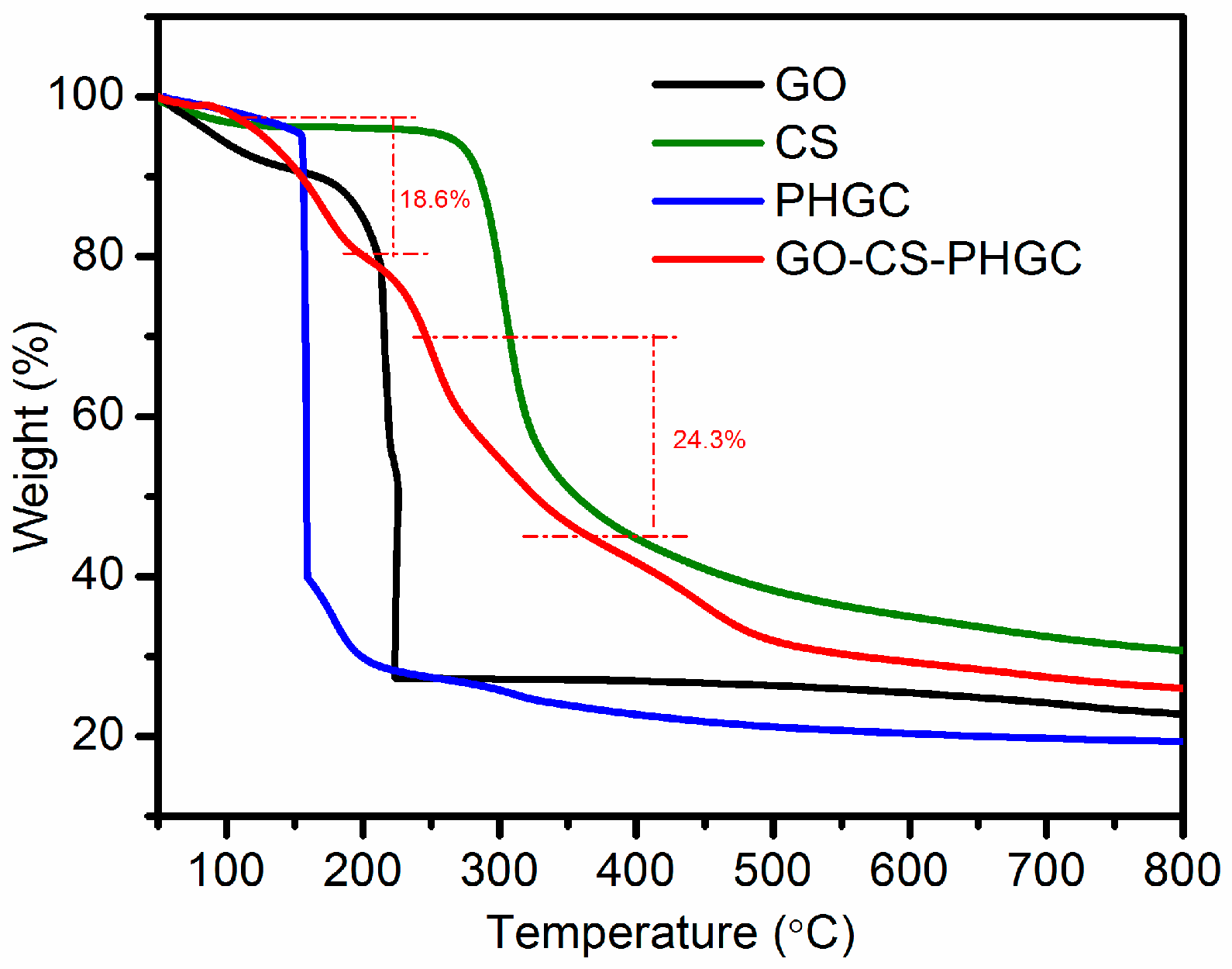
2.1.6. TGA Analysis
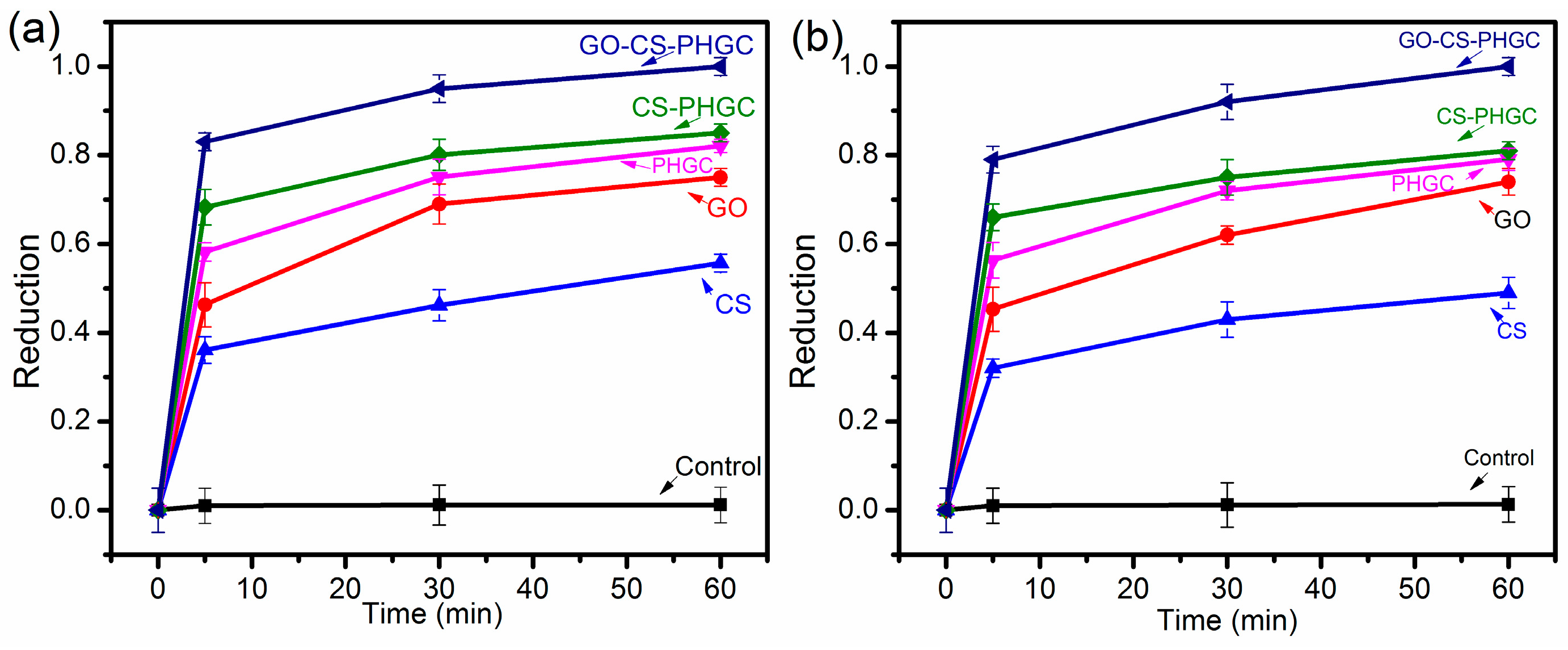
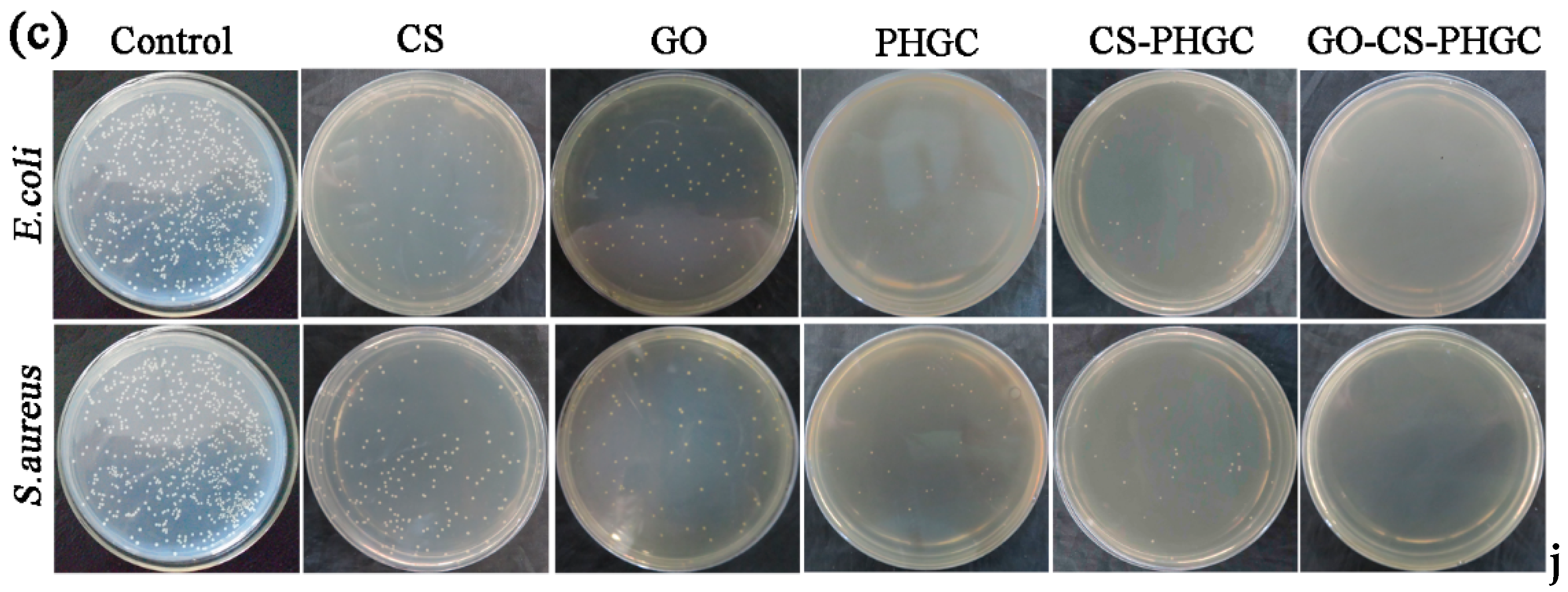
2.2. Antibacterial Test
2.2.1. Biocidal Kinetic Test
2.2.2. MIC Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO
3.3. Preparation of PHGC
3.4. Preparation of Chitosan-Guanidine Composites
3.5. Preparation of GO-CS-PHGC
3.6. Characterization
3.7. Bacterial Culture and Antibacterial Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Rottingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Kang, J.; Han, J.; Gao, Y.; Gao, T.; Lan, S.; Xiao, L.; Zhang, Y.; Gao, G.; Chokto, H.; Dong, A. Unexpected enhancement in antibacterial activity of N-halamine polymers from spheres to fibers. ACS Appl. Mater. Interfaces 2015, 7, 17516–17526. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.E.; Wei, L.; Goh, K.; Wiraja, C.; Liu, Z.; Xu, C.; Jiang, R.; Wei, J.; Chen, Y. Synergism of water shock and a biocompatible block copolymer potentiates the antibacterial activity of graphene oxide. Small 2016, 12, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Abdelhamid, H.N.; Wu, H.F. Near infrared (NIR) laser mediated surface activation of graphene oxide nanoflakes for efficient antibacterial, antifungal and wound healing treatment. Colloid Surf. B 2015, 127, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Cai, X.; Shi, Q.S.; Liu, L.L.; Wan, D.L.; Tan, S.Z.; Ouyang, Y.S. Poly-l-lysine-modified reduced graphene oxide stabilizes the copper nanoparticles with higher water-solubility and long-term additively antibacterial activity. Colloid Surf. B 2013, 107, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, M.A.; Othman, A.A.; Alsharaeh, E.H. Synthesis and characterization of the in situ bulk polymerization of PMMA containing graphene sheets using microwave irradiation. Molecules 2013, 18, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.M.S.; Mishra, S. Use of graphite oxide and graphene oxide as catalysts in the synthesis of dipyrromethane and calix[4]pyrrole. Molecules 2011, 16, 7256–7266. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, E.; Han, J.W.; Kim, J.H.; Gurunathan, S. A novel biomolecule-mediated reduction of graphene oxide: A multifunctional anti-cancer agent. Molecules 2016, 21, 375. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y.C. Graphene-based nanomaterials as heterogeneous acid catalysts: A comprehensive perspective. Molecules 2014, 19, 14582–14614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.P.; Li, Q.L.; Xiang, S.D.; Wang, Y.; Wang, C.Y.; Jiang, W.; Zhou, H.; Yang, Y.W.; Tang, J. Covalent modification of graphene oxide with polynorbornene by surface-initiated ring-opening metathesis polymerization. Polymer 2014, 55, 6044–6050. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials 2014, 35, 9963–9971. [Google Scholar] [CrossRef] [PubMed]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.X.; Tan, W.H. Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Liu, Z.M.; Ye, X.P.; Hu, K.; Zhong, H.Q.; Yuan, X.C.; Xiong, H.L.; Guo, Z.Y. A facile one-pot method to two kinds of graphene oxide-based hydrogels with broad-spectrum antimicrobial properties. Chem. Eng. J. 2015, 260, 331–337. [Google Scholar] [CrossRef]
- De Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Souza, A.G.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloid Surf. B 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Sreeprasad, T.S.; Maliyekkal, M.S.; Deepti, K.; Chaudhari, K.; Xavier, P.L.; Pradeep, T. Transparent, luminescent, antibacterial and patternable film forming composites of graphene oxide/reduced graphene oxide. ACS Appl. Mater. Interface 2011, 3, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Alsharaeh, E.; Mussa, Y.; Ahmed, F.; Aldawsari, Y.; Al-Hindawi, M.; Sing, G.K. Novel route for the preparation of cobalt oxide nanoparticles/reduced graphene oxide nanocomposites and their antibacterial activities. Ceram. Int. 2016, 42, 3407–3410. [Google Scholar] [CrossRef]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Ayob, M.K.; Huang, N.M.; Neoh, H.M.; Jamal, R. Antibacterial hybrid cellulose—Graphene oxide nanocomposite immobilized with silver nanoparticles. RSC Adv. 2015, 5, 26263–26268. [Google Scholar] [CrossRef]
- Wahid, M.H.; Stroeher, U.H.; Eroglu, E.; Chen, X.J.; Vimalanathan, K.; Raston, C.L.; Boulos, R.A. Aqueous based synthesis of antimicrobial-decorated graphene. J. Colloid Interface Sci. 2015, 443, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Wu, J.J.; Su, Y.; Zhou, J.; Gao, Y.; Yu, H.Y.; Gu, J.S. Layer-by-layer assembly of graphene oxide on polypropylene macroporous membranes via click chemistry to improve antibacterial and antifouling performance. Appl. Surf. Sci. 2015, 332, 300–307. [Google Scholar] [CrossRef]
- Soroush, A.; Ma, W.; Cyr, M.; Rahaman, M.S.; Asadishad, B.; Tufenkji, N. In situsilver decoration on graphene oxide-treated thin film composite forward osmosis membranes: Biocidal properties and regeneration potential. Environ. Sci. Technol. Lett. 2016, 3, 13–18. [Google Scholar] [CrossRef]
- Richtera, L.; Chudobova, D.; Cihalova, K.; Kremplova, M.; Milosavljevic, V.; Kopel, P.; Blazkova, I.; Hynek, D.; Adam, V.; Kizek, R. The composites of graphene oxide with metal or semimetal nanoparticles and their effect on pathogenic microorganisms. Materials 2015, 8, 2994–3011. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Q.; Xu, L.G.; Zhang, S.; Feng, L.Z.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene oxide-silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl. Mater. Interface 2013, 5, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Dellieu, L.; Lawaree, E.; Reckinger, N.; Didembourg, C.; Letesson, J.J.; Sarrazin, M.; Deparis, O.; Matroule, J.Y.; Deparis, J.F. Do CVD grown graphene films have antibacterial activity on metallic substrates? Carbon 2015, 84, 310–316. [Google Scholar] [CrossRef]
- Kavitha, T.; Gopalan, A.I.; Lee, K.P.; Park, S.Y. Glucose sensing, photocatalytic and antibacterial properties of graphene-ZnO nanoparticle hybrids. Carbon 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- Chang, Y.N.; Ou, X.M.; Zeng, G.M.; Gong, J.L.; Deng, C.H.; Jiang, Y.; Liang, J.; Yuan, G.Q.; Liu, H.Y.; He, X. Synthesis of magnetic graphene oxide-TiO2 and their antibacterial properties under solar irradiation. Appl. Surf. Sci. 2015, 343, 1–10. [Google Scholar] [CrossRef]
- Deng, C.H.; Gong, J.L.; Zeng, G.M.; Niu, C.G.; Niu, Q.Y.; Zhang, W.; Liu, H.Y. Inactivation performance and mechanism of Escherichia coli in aqueous system exposed to iron oxide loaded graphene nanocomposites. J. Hazard. Mater. 2014, 276, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.C.; Wang, Y.J.; Yan, X.L.; Sun, D.D. Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity. New J. Chem. 2011, 35, 1418–1423. [Google Scholar] [CrossRef]
- Wang, Y.W.; Cao, A.N.; Jiang, Y.; Zhang, I.; Liu, J.H.; Liu, Y.F.; Wang, H.F. Superior antibacterial activity of zinc oxide/graphene oxide composites localized around bacteria. ACS Appl. Mater. Interface 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Ray Chowdhuri, A.; Tripathy, S.; Chandra, S.; Roy, S.; Sahu, S.K. A ZnO decorated chitosan-graphene oxide nanocomposite shows significantly enhanced antimicrobial activity with ROS generation. RSC Adv. 2015, 5, 49420–49428. [Google Scholar] [CrossRef]
- Marta, B.; Potara, M.; Iliut, M.; Jakab, E.; Radu, T.; Imre-Lucaci, F.; Katona, G.; Popescu, O.; Astilean, S. Designing chitosan-silver nanoparticles-graphene oxide nanohybrids with enhanced antibacterial activity against Staphylococcus aureus. Colloid Surf. A 2015, 487, 113–120. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Chi, W.; Yu, C.; Yu, Y. Preparation and antibacterial properties of Ag@polydopamine/graphene oxide sheet nanocomposite. Appl. Surf. Sci. 2013, 282, 181–185. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, J.L.; Zeng, G.M.; Ou, X.M.; Chang, Y.N.; Deng, C.H.; Zhang, J.; Liu, H.Y.; Huang, S.Y. Magnetic chitosan-graphene oxide composite for anti-microbial and dye removal applications. Int. J. Biol. Macromol. 2016, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lin, M.S.; Tan, S.Z.; Mai, W.J.; Zhang, Y.M.; Liang, Z.W.; Lin, Z.D.; Zhang, X.J. The use of polyethyleneimine-modified reduced graphene oxide as a substrate for silver nanoparticles to produce a material with lower cytotoxicity and long-term antibacterial activity. Carbon 2012, 50, 3407–3415. [Google Scholar] [CrossRef]
- Tai, Z.X.; Ma, H.B.; Liu, B.; Yan, X.B.; Xue, Q.J. Facile synthesis of Ag/GNS-g-PAA nanohybrids for antimicrobial applications. Colloid Surf. B 2012, 89, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.Y.; Kim, S.Y.; Kim, H.G.; Moon, G.S.; In, I. Antibacterial activity of chemically reduced graphene oxide assembly with chitosan through noncovalent interactions. Chem. Lett. 2013, 42, 66–67. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Ostadhossein, F.; Simchi, A. Physicochemical and antibacterial properties of chitosan-polyvinylpyrrolidone films containing self-organized graphene oxide nanolayers. J. Appl. Polym. Sci. 2016, 133, 43194. [Google Scholar] [CrossRef]
- Wang, X.; Lu, P.; Li, Y.; Xiao, H.; Liu, X. Antibacterial activities and mechanisms of fluorinated graphene and guanidine-modified graphene. RSC Adv. 2016, 6, 8763–8772. [Google Scholar] [CrossRef]
- Guan, Y.; Xiao, H.; Sullivan, H.; Zheng, A. Antimicrobial-modified sulfite pulps prepared by in situ copolymerization. Carbohydr. Polym. 2007, 69, 688–696. [Google Scholar] [CrossRef]
- Mitra, S.; Kandambeth, S.; Biswal, B.P.; Khayum, M.A.; Choudhury, C.K.; Mehta, M.; Kaur, G.; Banerjee, S.; Prabhune, A.; Verma, S.; et al. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs). J. Am. Chem. Soc. 2016, 138, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Jiang, J.M.; Chen, Y.M. Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polymer 1999, 40, 6189–6198. [Google Scholar] [CrossRef]
- Liu, S.B.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H.J. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, S.Y.; Dong, A.; Hao, Y.P.; Shi, S.Q.; Sun, Z.J.; Gao, G.; Chen, Y.X. Developing of a novel antibacterial agent by functionalization of graphene oxide with guanidine polymer with enhanced antibacterial activity. Appl. Surf. Sci. 2015, 355, 446–452. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H.; Li, A.M.; Cheng, R.S. pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Chem. Eng. J. 2016, 284, 1397–1405. [Google Scholar] [CrossRef]
- Wongkongkatep, P.; Manopwisedjaroen, K.; Tiposoth, P.; Archakunakorn, S.; Pongtharangkul, T.; Suphantharika, M.; Honda, K.; Hamachi, I.; Wongkongkatep, J. Bacteria interface pickering emulsions stabilized by self-assembled bacteria-chitosan network. Langmuir 2012, 28, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zhu, J.A.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Sahariah, P.; Oskarsson, B.M.; Hjalmarsdottir, M.A.; Masson, M. Synthesis of guanidinylated chitosan with the aid of multiple protecting groups and investigation of antibacterial activity. Carbohydr. Polym. 2015, 127, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cai, J.; Du, Y.; Lin, J.; Wang, C.; Xiong, K. Preparation and anti-TMV activity of guanidinylated chitosan hydrochloride. J. Appl. Polym. Sci. 2009, 112, 3522–3528. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Li, X.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Farani, M.R.; Dehghani, M.; Tamjid, E.; Simchi, A. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity. Carbon 2015, 84, 91–102. [Google Scholar] [CrossRef]
- Lim, H.N.; Huang, N.M.; Loo, C.H. Facile preparation of graphene-based chitosan films: Enhanced thermal, mechanical and antibacterial properties. J. Non-Cryst Solids 2012, 358, 525–530. [Google Scholar] [CrossRef]
- Mazaheri, M.; Akhavan, O.; Simchi, A. Flexible bactericidal graphene oxide-chitosan layers for stem cell proliferation. Appl. Surf. Sci. 2014, 301, 456–462. [Google Scholar] [CrossRef]
- Xie, C.M.; Lu, X.; Han, L.; Xu, J.L.; Wang, Z.M.; Jiang, L.L.; Wang, K.F.; Zhang, H.P.; Ren, F.Z.; Tang, Y.H. Biomimetic mineralized hierarchical graphene oxide/chitosan scaffolds with adsorbability for immobilization of nanoparticles for biomedical applications. ACS Appl. Mater. Interface 2016, 8, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Travlou, N.A.; Deliyanni, E.A. The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions. Colloids Surf. B Biointerfaces 2014, 113, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Tu, Y.F.; Li, L.A.; Shang, S.M.; Tao, X.M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interface 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Z.; Mu, C.; Zhu, W.; Yan, X.; Hu, X.; Yang, J. Flexible polyurethane composites prepared by incorporation of polyethylenimine-modified slightly reduced graphene oxide. Carbon 2016, 98, 432–440. [Google Scholar] [CrossRef]
- Sun, X.F.; Qin, J.; Xia, P.F.; Guo, B.B.; Yang, C.M.; Song, C.; Wang, S.G. Graphene oxide-silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Qian, L.; Xiao, H.; Zhao, G.; He, B. Synthesis of modified guanidine-based polymers and their antimicrobial activities revealed by AFM and CLSM. ACS Appl. Mater. Interfaces 2011, 3, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Sample Availability: Not available.




| Sample | GO-CS-PHGC | GO + (CS-PHGC) | GO + CS + PHGC |
|---|---|---|---|
| MIC (μg/mL) | 32 | 128 | 256 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Gao, Y.; Sun, Z.; Chang, D.; Gao, G.; Dong, A. Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites. Molecules 2017, 22, 12. https://doi.org/10.3390/molecules22010012
Li P, Gao Y, Sun Z, Chang D, Gao G, Dong A. Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites. Molecules. 2017; 22(1):12. https://doi.org/10.3390/molecules22010012
Chicago/Turabian StyleLi, Ping, Yangyang Gao, Zijia Sun, Dan Chang, Ge Gao, and Alideertu Dong. 2017. "Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites" Molecules 22, no. 1: 12. https://doi.org/10.3390/molecules22010012





