Ginsenoside Rg3 Improves Recovery from Spinal Cord Injury in Rats via Suppression of Neuronal Apoptosis, Pro-Inflammatory Mediators, and Microglial Activation
Abstract
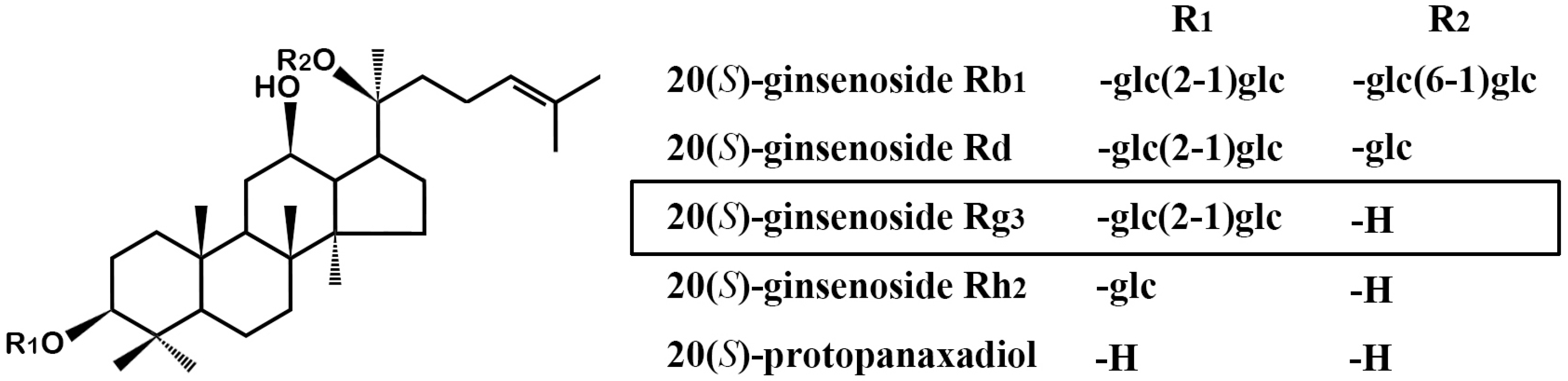
:1. Introduction
2. Results
2.1. GRg3 Improves Behavioral Motor Functions of Rats Subjected to Compressive SCI
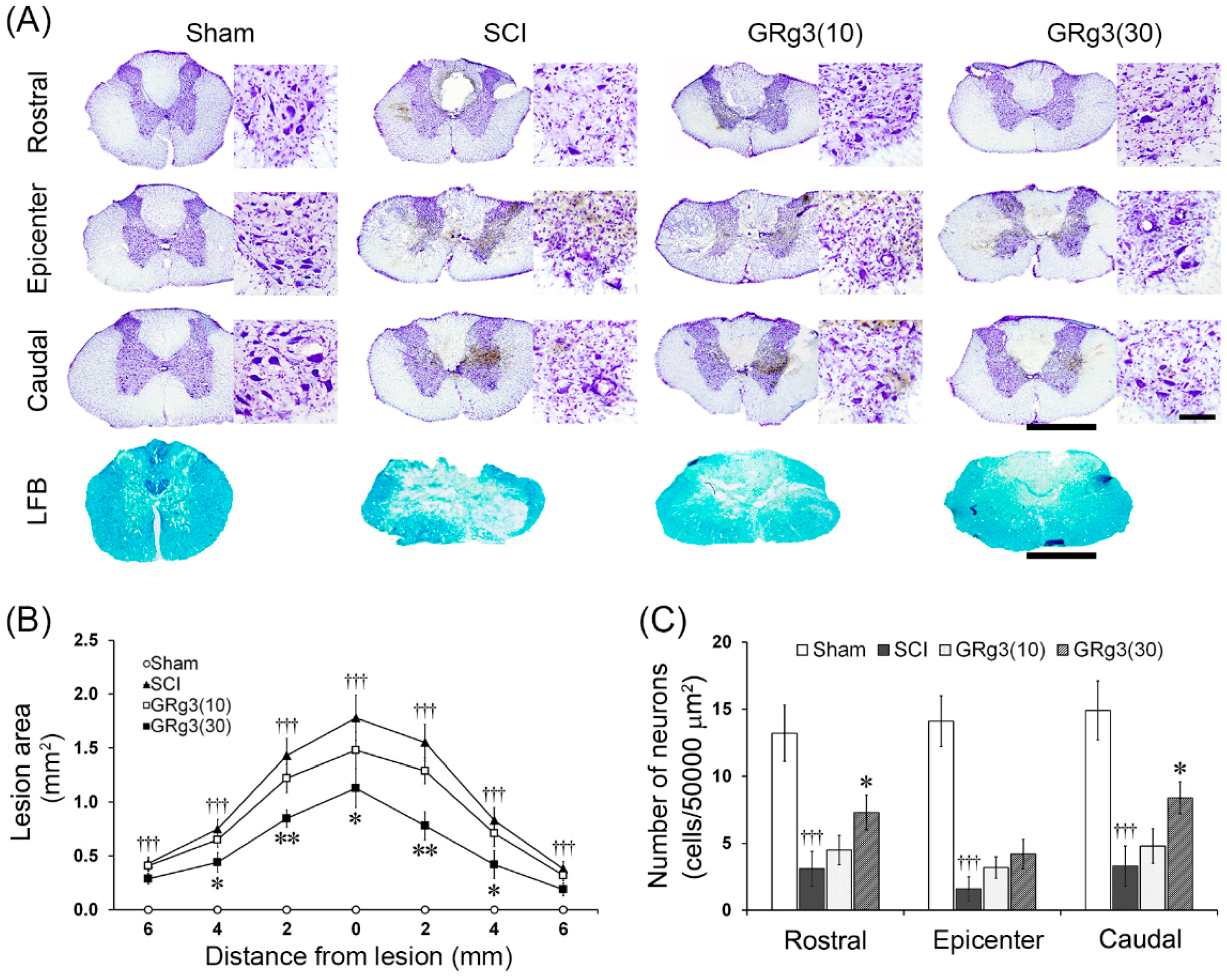
2.2. GRg3 Reduces Spinal Tissue Damage of Rats Subjected to Compressive SCI
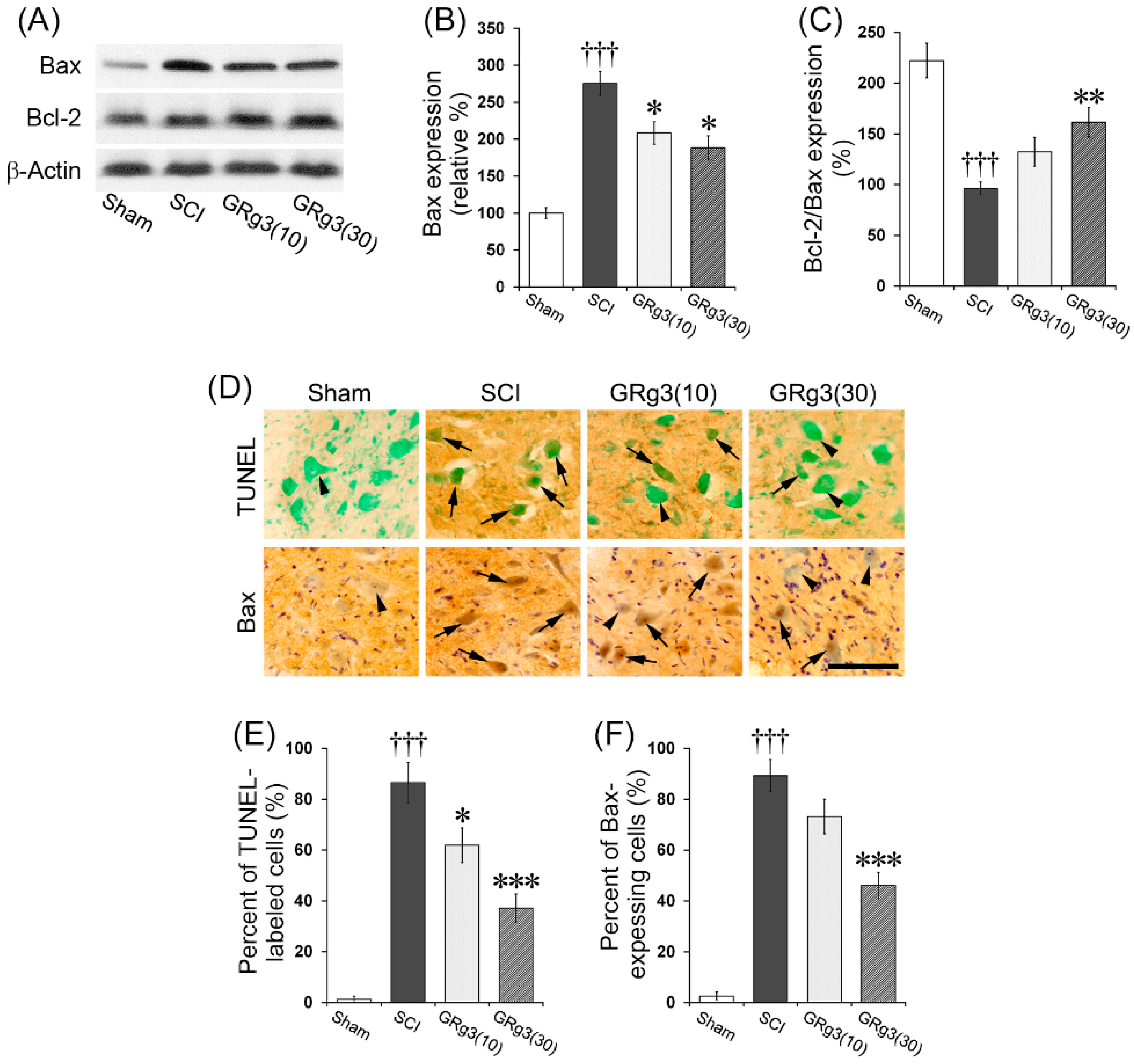
2.3. GRg3 Attenuates Neuronal Apoptosis in the Spinal Cord
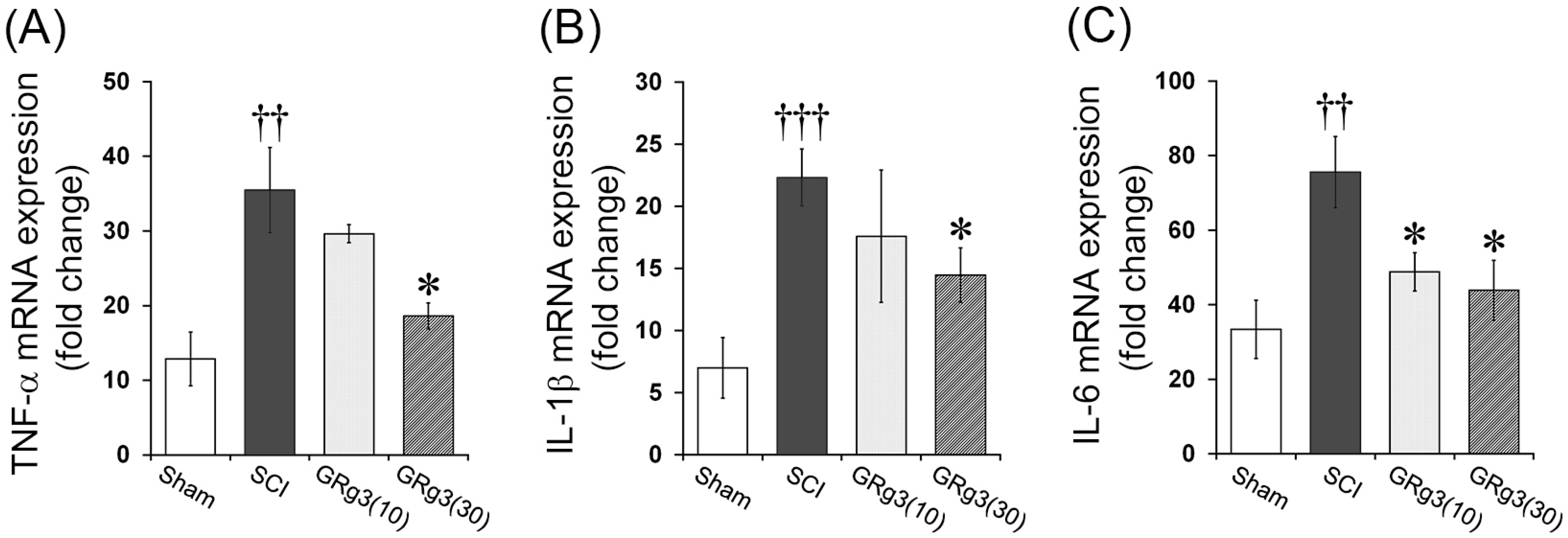
2.4. GRg3 Attenuates TNF-α, IL-1β, and IL-6 mRNA Expression in the Spinal Cord
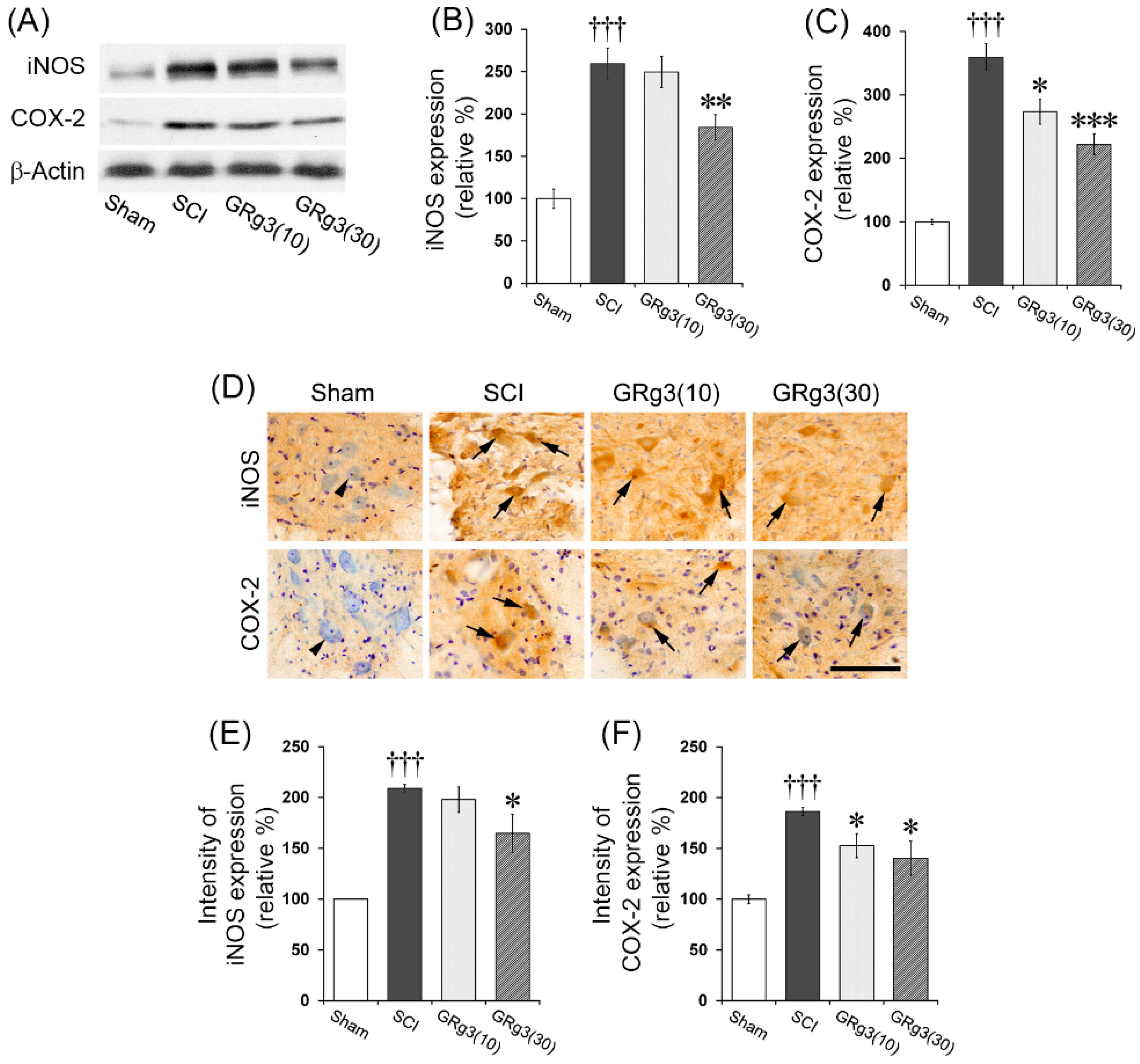
2.5. GRg3 Attenuates iNOS and COX-2 Expressions in the Spinal Cord
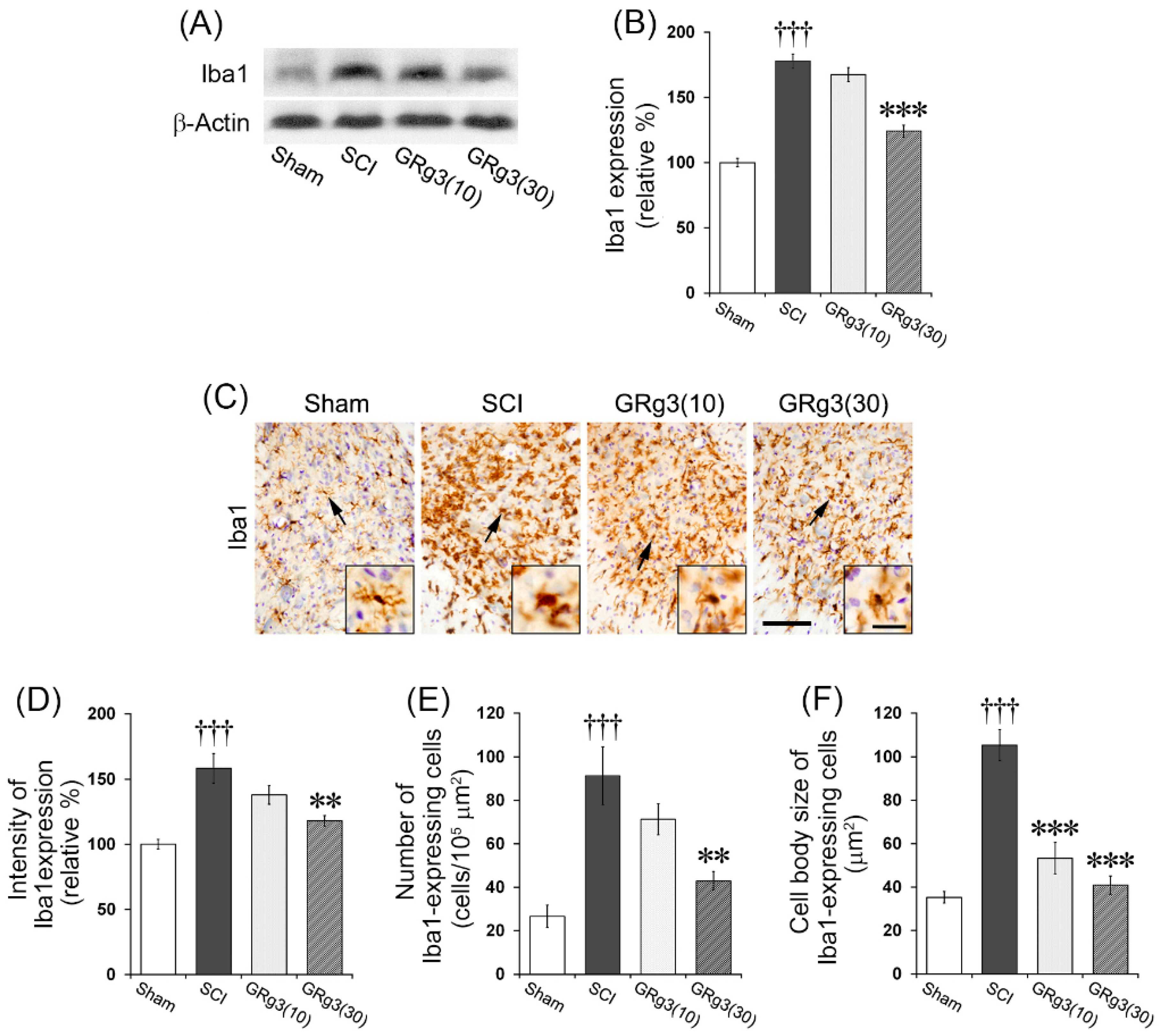
2.6. GRg3 Suppresses Microglial Activation in the Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Experimental Groups and Drug Treatment
4.4. Spinal Cord Compression Injury
4.5. Behavioral Assessment
4.5.1. BBB Test
4.5.2. Inclined Plane Test
4.5.3. Toe Spread and Hind Foot Bar Grab Tests
4.6. Spinal Cord Tissue Preparation and Histological Assessment
4.7. Immunoshistochemistry
4.8. Quantitative Real-Time PCR Measurement
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, Demographics, and Pathophysiology of Acute Spinal Cord Injury. Spine (Phila Pa. 1976) 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Beattie, M.S. Inflammation and Apoptosis: Linked Therapeutic Targets in Spinal Cord Injury. Trends Mol. Med. 2004, 10, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative Stress in Spinal Cord Injury and Antioxidant-Based Intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Lai, B.Q.; Zeng, X.; Che, M.T.; Ling, E.A.; Wu, W.; Zeng, Y.S. Cell Transplantation and Neuroengineering Approach for Spinal Cord Injury Treatment: A Summary of Current Laboratory Findings and Review of Literature. Cell Transplant. 2016, 25, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Sheth, S.; Zustiak, S.P. Polymeric Particle-Mediated Molecular Therapies to Treat Spinal Cord Injury. Int. J. Pharm. 2016, 516, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, H.; An, J.; Zhang, R.; Chen, B.; Hao, D.J. Therapeutic Effects of Traditional Chinese Medicine on Spinal Cord Injury: A Promising Supplementary Treatment in Future. Evid. Based Complement. Altern. Med. 2016, 2016, 8958721. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Kim, Y.; Lee, K.; Na, S.W.; Hong, S.P.; Valan Arasu, M.; Yoon, Y.W.; Kim, J. Panax Ginseng Improves Functional Recovery after Contusive Spinal Cord Injury by Regulating the Inflammatory Response in Rats: An in Vivo Study. Evid. Based Complement. Altern. Med. 2015, 2015, 817096. [Google Scholar]
- Wang, W.; Shen, H.; Xie, J.J.; Ling, J.; Lu, H. Neuroprotective Effect of Ginseng against Spinal Cord Injury Induced Oxidative Stress and Inflammatory Responses. Int. J. Clin. Exp. Med. 2015, 8, 3514–3521. [Google Scholar] [PubMed]
- Huang, F.; Li, Y.N.; Yin, F.; Wu, Y.T.; Zhao, D.X.; Li, Y.; Zhang, Y.F.; Zhu, Q.S. Ginsenoside Rb1 Inhibits Neuronal Apoptosis and Damage, Enhances Spinal Aquaporin 4 Expression and Improves Neurological Deficits in Rats with Spinal Cord Ischemia-reperfusion Injury. Mol. Med. Rep. 2015, 11, 3565–3572. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, Q.; Man, X.; Guo, L.; Hao, L. Ginsenoside Rd Inhibits Apoptosis Following Spinal Cord ischemia/reperfusion Injury. Neural Regen. Res. 2014, 9, 1678–1687. [Google Scholar] [PubMed]
- Cong, L.; Chen, W. Neuroprotective Effect of Ginsenoside Rd in Spinal Cord Injury Rats. Basic Clin. Pharmacol. Toxicol. 2016, 119, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Williamson, E.M.; Putnam, S.; Farrimond, J.; Whalley, B.J. Effects and Mechanisms of Ginseng and Ginsenosides on Cognition. Nutr. Rev. 2014, 72, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Park, J.; Kim, S.H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Ginsenoside rg3 Alleviates Lipopolysaccharide-Induced Learning and Memory Impairments by Anti-Inflammatory Activity in Rats. Biomol. Ther. (Seoul) 2013, 21, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Jung, H.J.; Choi, W.Y.; Lim, C.J. Antioxidative, Anti-Inflammatory, and Matrix Metalloproteinase Inhibitory Activities of 20(S)-Ginsenoside Rg3 in Cultured Mammalian Cell Lines. Mol. Biol. Rep. 2013, 40, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; You, J.M.; Yun, S.J.; Son, M.S.; Nam, K.N.; Hong, J.W.; Kim, S.Y.; Choi, S.Y.; Lee, E.H. Ginsenoside rb1 and rg3 Attenuate Glucocorticoid-Induced Neurotoxicity. Cell. Mol. Neurobiol. 2010, 30, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Park, J.Y.; Choi, S.; Lee, J.B.; Jung, H.; Kim, T.D.; Yoon, S.R.; Choi, I.; Shim, S.; Park, Y.J. Ginsenoside Rg3 Regulates S-Nitrosylation of the NLRP3 Inflammasome Via Suppression of iNOS. Biochem. Biophys. Res. Commun. 2015, 463, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, S.; Li, G.; Liu, Z.; Xu, B. 20(S)-Ginsenoside Rg3, a Neuroprotective Agent, Inhibits Mitochondrial Permeability Transition Pores in Rat Brain. Phytother. Res. 2009, 23, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Choi, M.S.; Sohn, N.W.; Shin, J.W. Ginsenoside Rg3 Attenuates Microglia Activation Following Systemic Lipopolysaccharide Treatment in Mice. Biol. Pharm. Bull. 2012, 35, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ahn, K.; Oh, T.H.; Nah, S.Y.; Rhim, H. Inhibitory Effect of Ginsenosides on NMDA Receptor-Mediated Signals in Rat Hippocampal Neurons. Biochem. Biophys. Res. Commun. 2002, 296, 247–254. [Google Scholar] [CrossRef]
- Kim, S.; Nah, S.Y.; Rhim, H. Neuroprotective Effects of Ginseng Saponins against L-Type Ca2+ Channel-Mediated Cell Death in Rat Cortical Neurons. Biochem. Biophys. Res. Commun. 2008, 365, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, F.; Geng, M.; Jiang, Y.; Yang, J.; Jiang, W.; Wang, C.; Liu, K. Neuroprotective Effect of 20(S)-Ginsenoside Rg3 on Cerebral Ischemia in Rats. Neurosci. Lett. 2005, 374, 92–97. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chen, P.; Yang, J.; Yun, Y.; Zhang, X.; Yang, R.; Shen, Z. Neuroprotective Effect of 20(R)-Ginsenoside Rg(3) Against Transient Focal Cerebral Ischemia in Rats. Neurosci. Lett. 2012, 526, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Kesslak, J.P.; Keirstead, H.S. Assessment of Behavior in Animal Models of Spinal Cord Injury. J. Spinal Cord Med. 2003, 26, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded Histological and Locomotor Outcomes after Spinal Cord Contusion using the NYU Weight-Drop Device Versus Transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Basso, D.M.; Walters, P.; Stokes, B.T.; Jakeman, L.B. Behavioral and Histological Outcomes Following Graded Spinal Cord Contusion Injury in the C57Bl/6 Mouse. Exp. Neurol. 2001, 169, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.S.; Hermann, G.E.; Rogers, R.C.; Bresnahan, J.C. Cell Death in Models of Spinal Cord Injury. Prog. Brain Res. 2002, 137, 37–47. [Google Scholar] [PubMed]
- Crowe, M.J.; Bresnahan, J.C.; Shuman, S.L.; Masters, J.N.; Beattie, M.S. Apoptosis and Delayed Degeneration after Spinal Cord Injury in Rats and Monkeys. Nat. Med. 1997, 3, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Kim, J.H.; Cho, Y.L.; Maeng, Y.S.; Lee, S.J.; Pyun, B.J.; Kim, Y.M.; Park, J.H.; Kwon, Y.G. 20(S)-Ginsenoside Rg3 Prevents Endothelial Cell Apoptosis via Inhibition of a Mitochondrial Caspase Pathway. Biochem. Biophys. Res. Commun. 2006, 349, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Z.; Sun, B.; Xu, J.; Jiang, J.; Luo, M. Ginsenoside Rg3 Attenuates Myocardial ischemia/reperfusion Injury via Akt/endothelial Nitric Oxide Synthase Signaling and the Bcell lymphoma/Bcell Lymphoma associated X Protein Pathway. Mol. Med. Rep. 2015, 11, 4518–4524. [Google Scholar] [PubMed]
- Bartholdi, D.; Schwab, M.E. Expression of Pro-Inflammatory Cytokine and Chemokine mRNA upon Experimental Spinal Cord Injury in Mouse: An in Situ Hybridization Study. Eur. J. Neurosci. 1997, 9, 1422–1438. [Google Scholar] [PubMed]
- Kwon, B.K.; Tetzlaff, W.; Grauer, J.N.; Beiner, J.; Vaccaro, A.R. Pathophysiology and Pharmacologic Treatment of Acute Spinal Cord Injury. Spine J. 2004, 4, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Miscusi, M.; Cardali, S.; Germano, A.; Suzuki, H.; Cuzzocrea, S.; Tomasello, F. Nitric Oxide in the Injured Spinal Cord: Synthases Cross-Talk, Oxidative Stress and Inflammation. Brain Res. Rev. 2007, 54, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Matsuyama, Y.; Nakashima, S.; Yanase, M.; Kiuchi, K.; Ishiguro, N. Effects of MPSS and a Potent iNOS Inhibitor on Traumatic Spinal Cord Injury. Neuroreport 2004, 15, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vales, R.; Garcia-Alias, G.; Guzman-Lenis, M.S.; Fores, J.; Casas, C.; Navarro, X.; Verdu, E. Effects of COX-2 and iNOS Inhibitors Alone Or in Combination with Olfactory Ensheathing Cell Grafts After Spinal Cord Injury. Spine (Phila Pa. 1976) 2006, 31, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Greenhalgh, A.D.; Kroner, A. Macrophage and Microglial Plasticity in the Injured Spinal Cord. Neuroscience 2015, 307, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Yenari, M.A. The Role of the Microglia in Acute CNS Injury. Metab. Brain Dis. 2015, 30, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Vilalta, A. How Microglia Kill Neurons. Brain Res. 2015, 1628, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Guan, Z.; McGaughy, V.; Fisher, L.; Hickey, W.F.; Basso, D.M. The Neuropathological and Behavioral Consequences of Intraspinal microglial/macrophage Activation. J. Neuropathol. Exp. Neurol. 2002, 61, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Guan, Z.; Wei, P.; Huitinga, I.; van Rooijen, N.; Stokes, B.T. Depletion of Hematogenous Macrophages Promotes Partial Hindlimb Recovery and Neuroanatomical Repair after Experimental Spinal Cord Injury. Exp. Neurol. 1999, 158, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Nystrom, B.; Berglund, J.E. Spinal Cord Restitution Following Compression Injuries in Rats. Acta Neurol. Scand. 1988, 78, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, A.S.; Tator, C.H. Objective Clinical Assessment of Motor Function after Experimental Spinal Cord Injury in the Rat. J. Neurosurg. 1977, 47, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kerasidis, H.; Wrathall, J.R.; Gale, K. Behavioral Assessment of Functional Deficit in Rats with Contusive Spinal Cord Injury. J. Neurosci. Methods 1987, 20, 167–179. [Google Scholar] [CrossRef]
- Sample Availability: Not available.

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-K.; Kweon, K.-J.; Kim, P.; Kim, H.-J.; Kim, S.-S.; Sohn, N.-W.; Maeng, S.; Shin, J.-W. Ginsenoside Rg3 Improves Recovery from Spinal Cord Injury in Rats via Suppression of Neuronal Apoptosis, Pro-Inflammatory Mediators, and Microglial Activation. Molecules 2017, 22, 122. https://doi.org/10.3390/molecules22010122
Kim D-K, Kweon K-J, Kim P, Kim H-J, Kim S-S, Sohn N-W, Maeng S, Shin J-W. Ginsenoside Rg3 Improves Recovery from Spinal Cord Injury in Rats via Suppression of Neuronal Apoptosis, Pro-Inflammatory Mediators, and Microglial Activation. Molecules. 2017; 22(1):122. https://doi.org/10.3390/molecules22010122
Chicago/Turabian StyleKim, Dong-Kyu, Ki-Jung Kweon, Pyungsoo Kim, Hee-Jung Kim, Sung-Soo Kim, Nak-Won Sohn, Sungho Maeng, and Jung-Won Shin. 2017. "Ginsenoside Rg3 Improves Recovery from Spinal Cord Injury in Rats via Suppression of Neuronal Apoptosis, Pro-Inflammatory Mediators, and Microglial Activation" Molecules 22, no. 1: 122. https://doi.org/10.3390/molecules22010122




