Penduliflaworosin, a Diterpenoid from Croton crassifolius, Exerts Anti-Angiogenic Effect via VEGF Receptor-2 Signaling Pathway
Abstract
:1. Introduction
2. Results
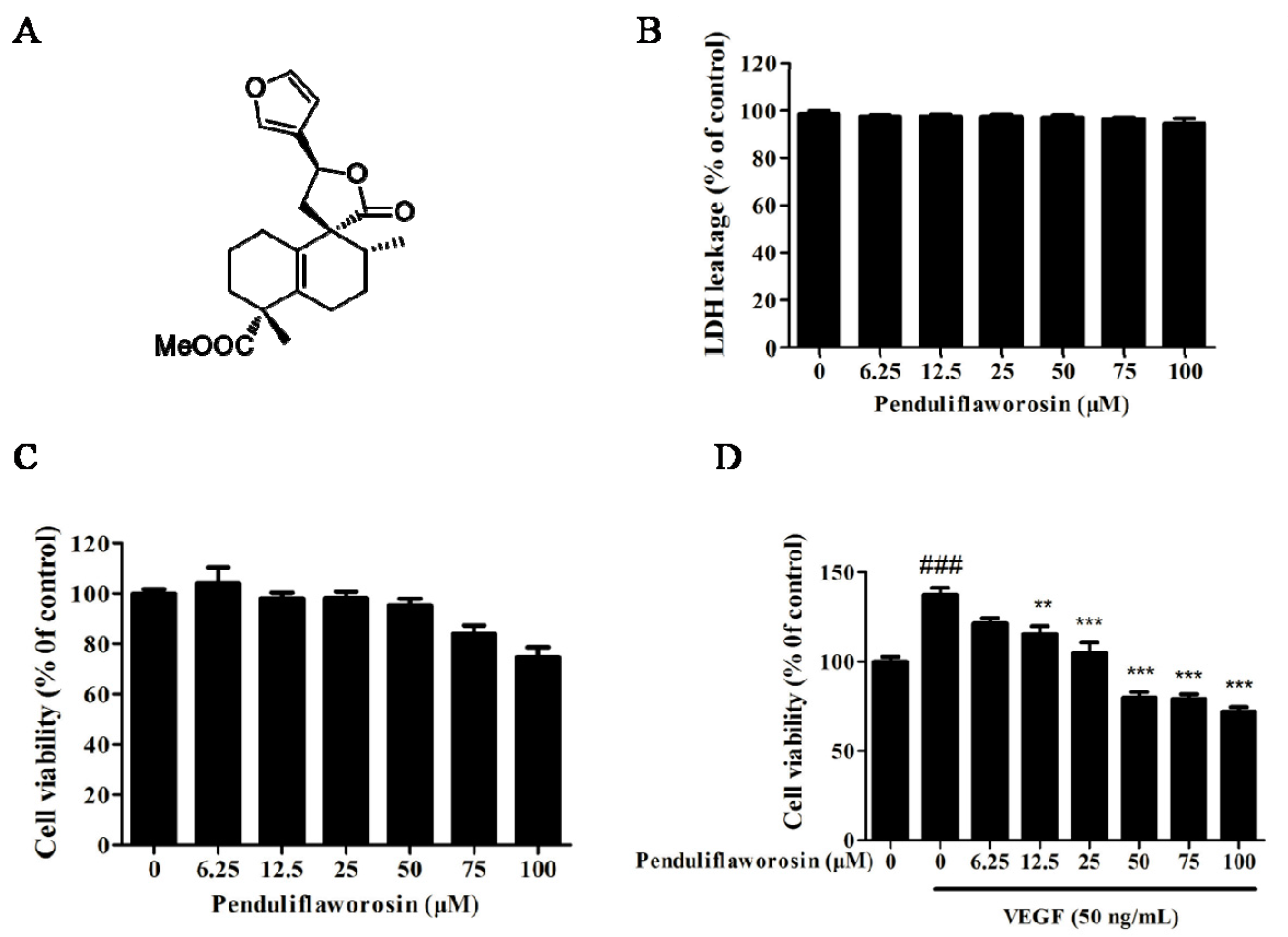
2.1. Penduliflaworosin Inhibited VEGF-Induced Proliferation of HUVECs
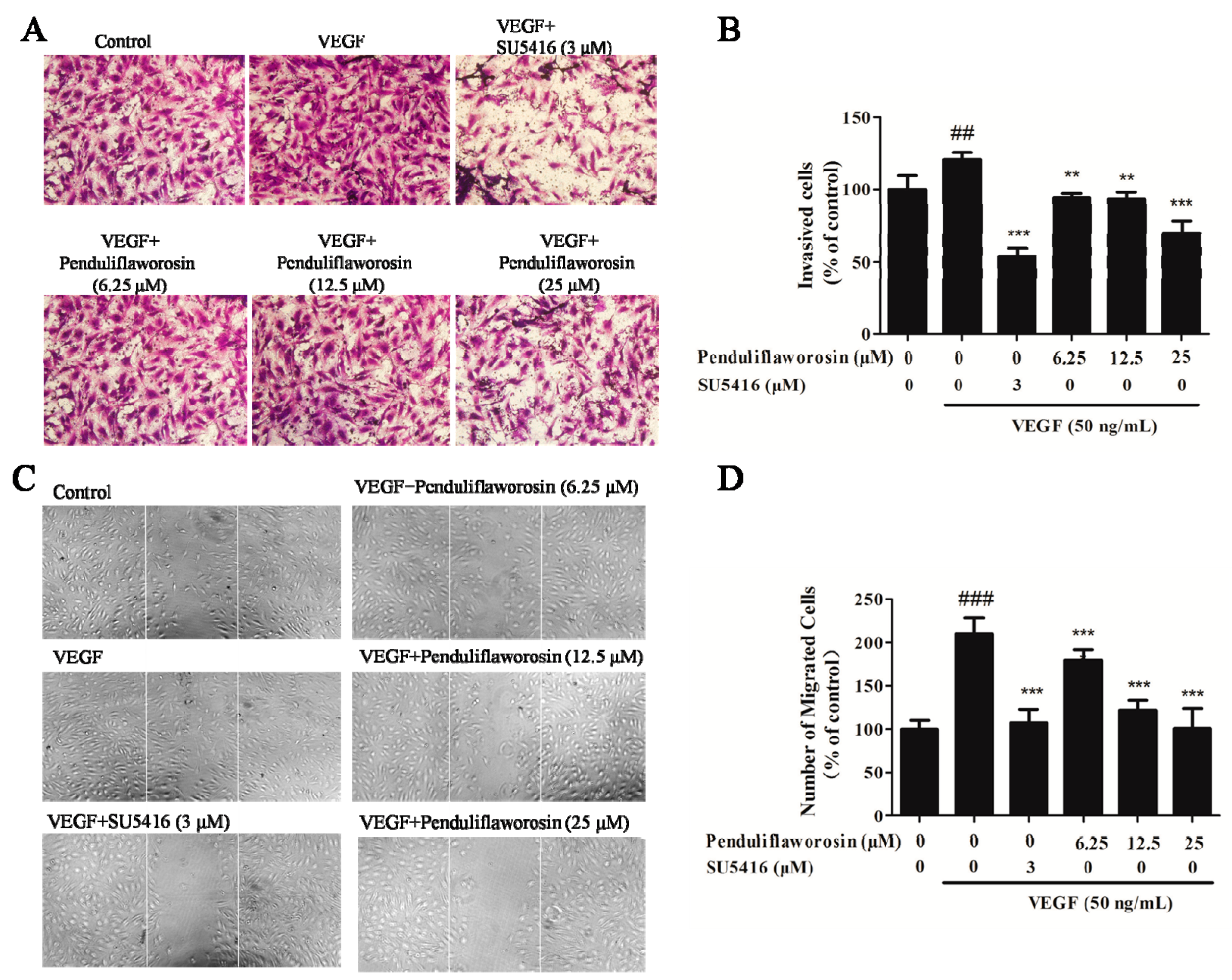
2.2. Penduliflaworosin Inhibited VEGF-Induced Migration, Invasion, and Tube Formation of HUVECs
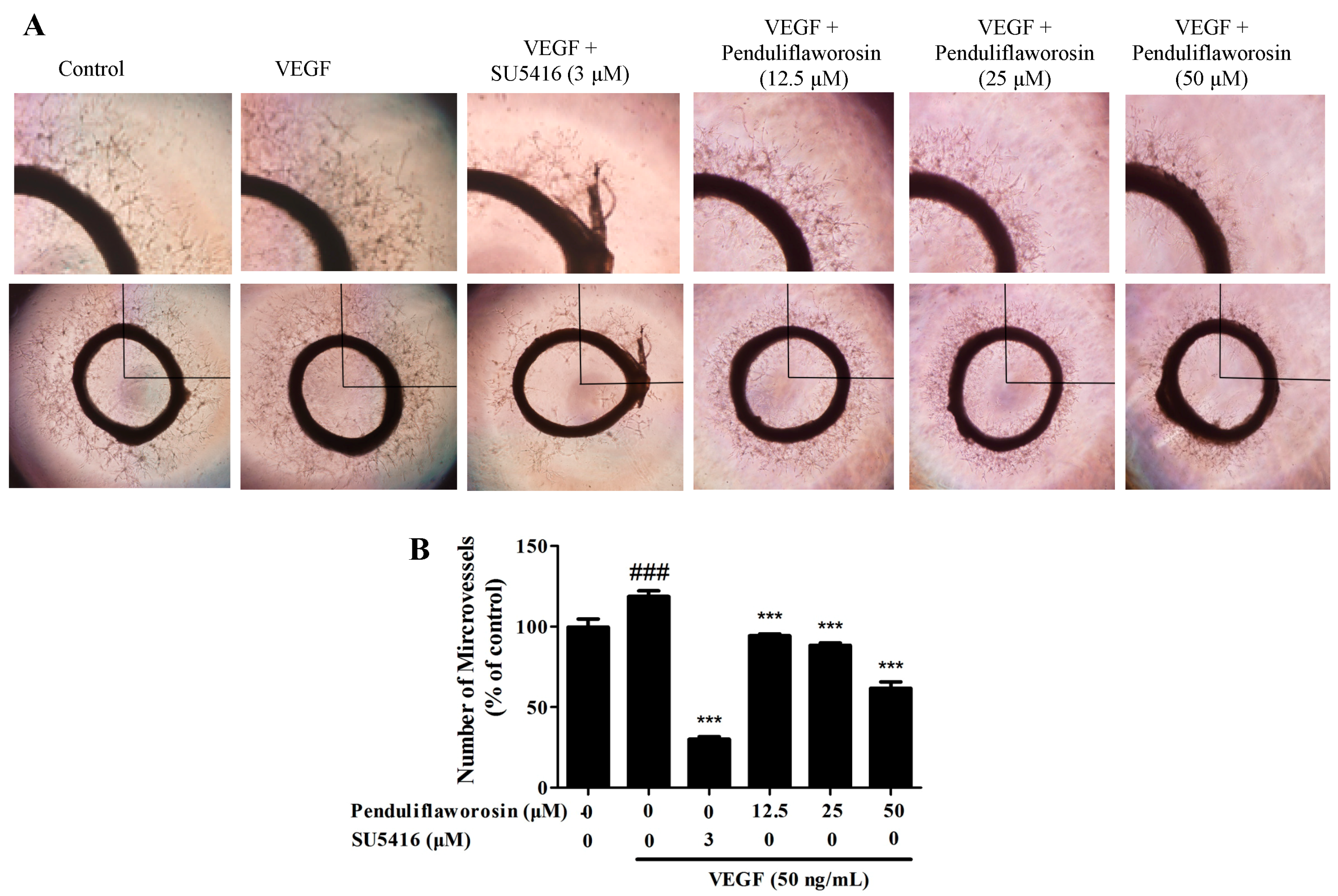
2.3. Penduliflaworosin Inhibited VEGF-Induced Vessel Sprout Formation
2.4. Penduliflaworosin Inhibited VEGF-Induced Blood Vessel Formation in Mice
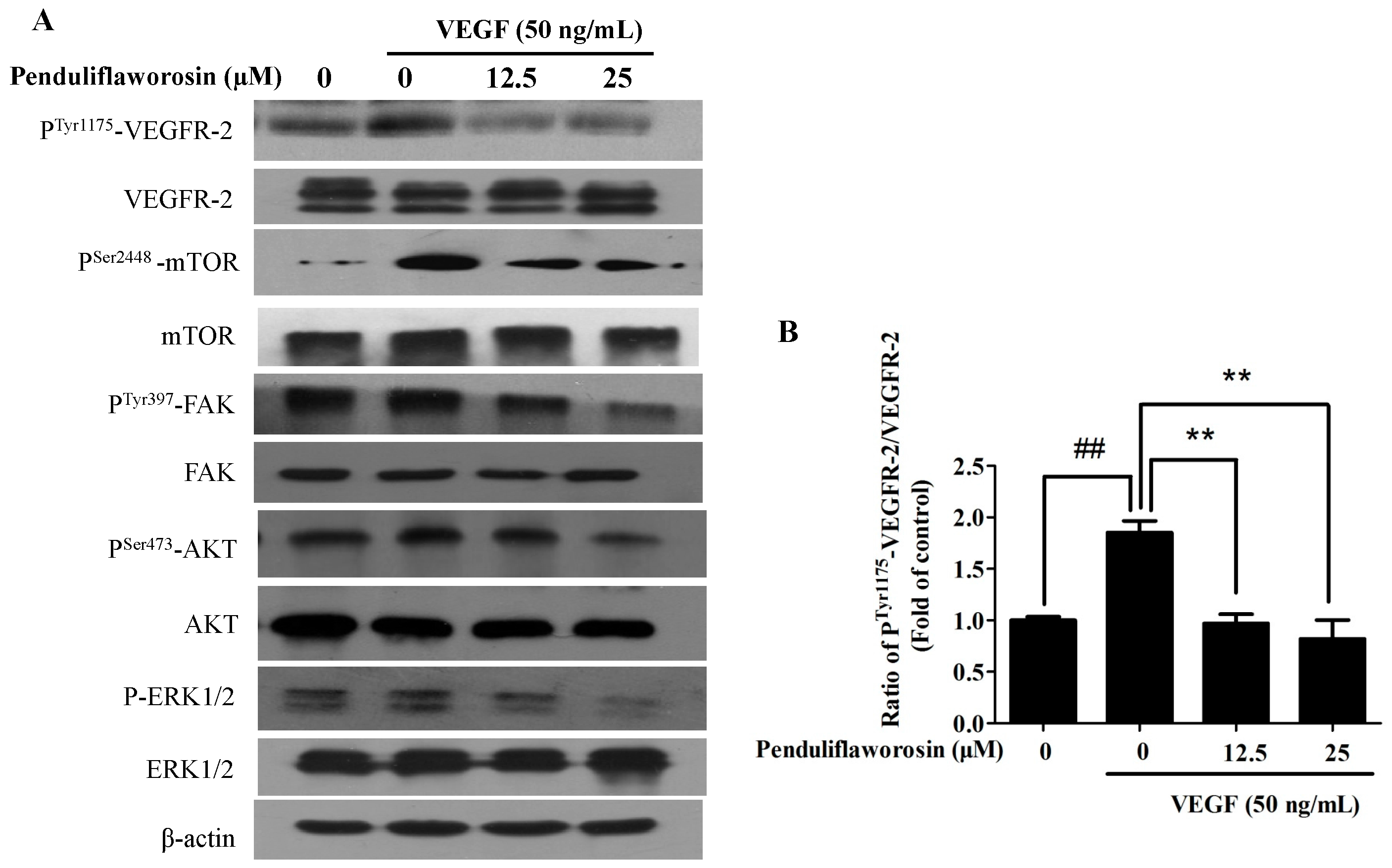
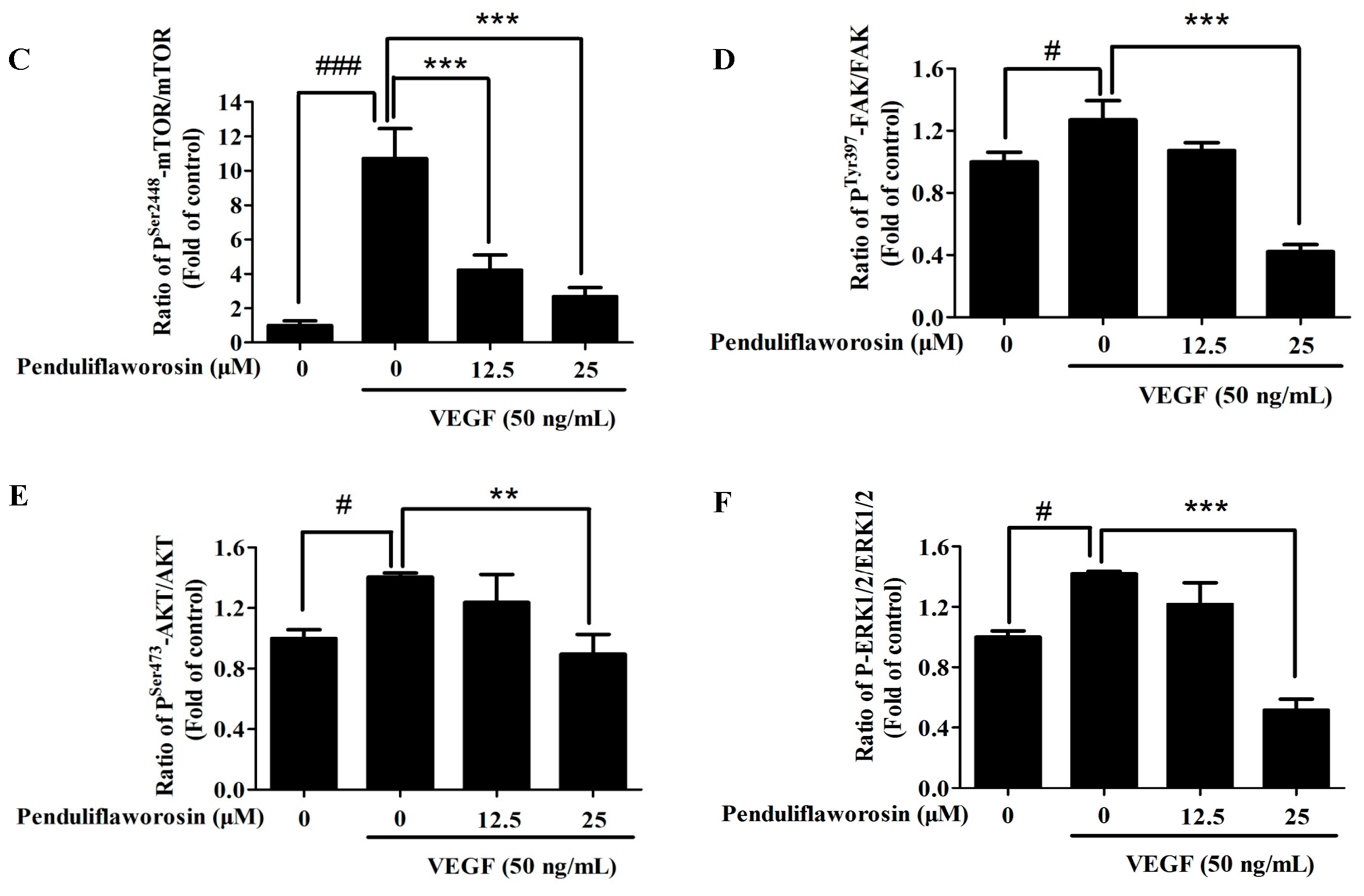
2.5. Penduliflaworosin Attenuated VEGF Receptor-2 Tyrosine Kinase Activity and the VEGF Receptor-2 Signaling Pathway
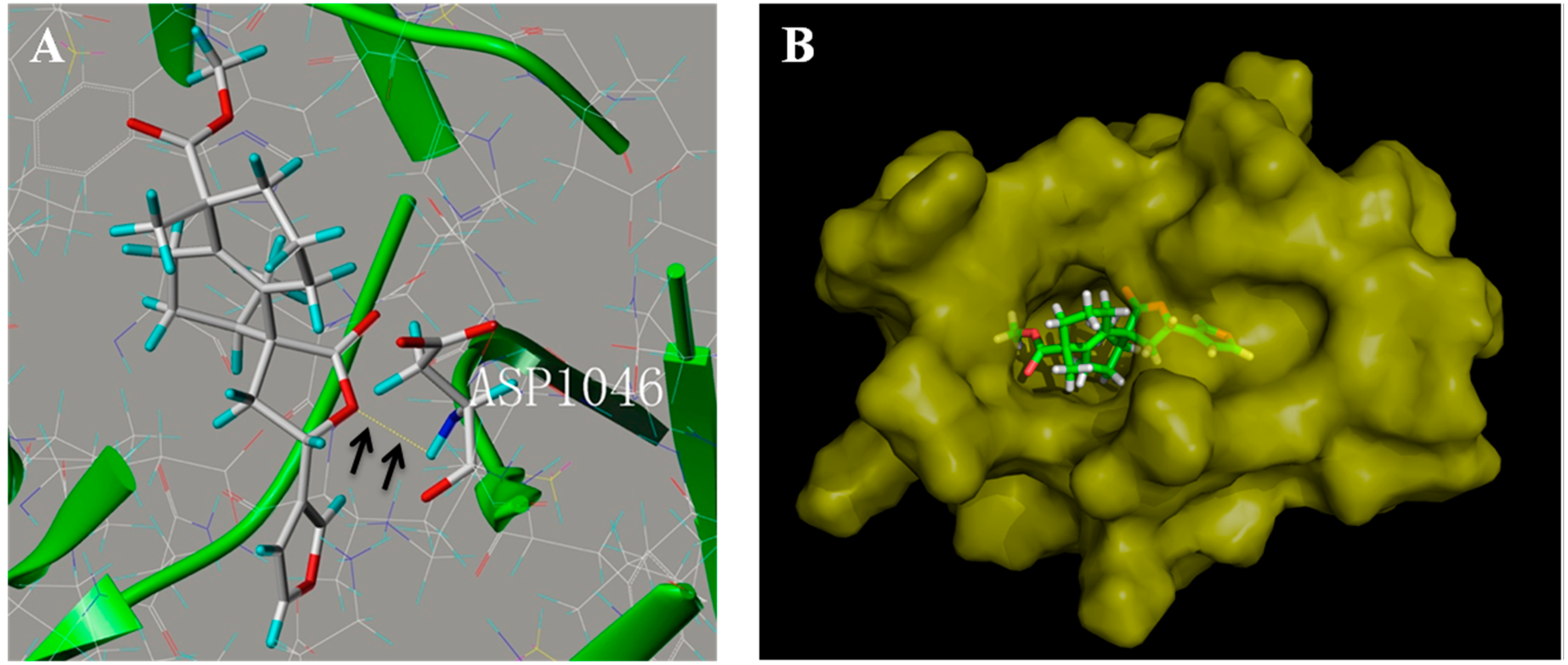
2.6. Penduliflaworosin Bound the ATP-Binding Sites of the VEGFR-2 Kinase Domain
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animals
4.3. LDH Toxicity Assay
4.4. Cell-Counting Assay
4.5. Wound-Healing Assay
4.6. Invasion Assay
4.7. Tube Formation Assay
4.8. Aortic Ring Assay
4.9. Matrigel Plug Assay
4.10. Western Blotting
4.11. Molecular Docking
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Michael, P.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol.-Cell. Physiol. 2002, 282, C947–C970. [Google Scholar]
- David, B.; Harold, W. Targeting the growth factors and angiogenesis pathways: Small molecules in solid tumors. J. Surg. Oncol. 2011, 103, 574–586. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, G.A.; Hurwitz, H.; Fehrenbacher, L.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Kabbinavar, F.; Holmgren, E.; Novotny, W. Bevacizumab plus irinotecan/5-FU/leucovorin for treatment of metastatic colorectal cancer results in survival benefit in all pre-specified patient subgroups. J. Clin. Oncol. 2004, 22, 3617. [Google Scholar]
- Miller, K.D.; Chap, L.I.; Holmes, F.A.; Cobleigh, M.A.; Marcom, P.K.; Fehrenbacher, L.; Dickler, M.; Overmoyer, B.A.; Reimann, J.D.; Sing, A.P. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 2005, 23, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Alan, S.; Robert, G.; Perry, M.C.; Julie, B.; Schiller, J.H.; Afshin, D.; Rogerio, L.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar]
- Patard, J.J.; Pignot, G.; Escudier, B.; Eisen, T.; Bex, A.; Sternberg, C.; Rini, B.; Roigas, J.; Choueiri, T.; Bukowski, R.; et al. ICUD-EAU international consultation on kidney cancer 2010: Treatment of metastatic disease. Eur. Urol. 2011, 60, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; Lecouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.M.; Figg, W.D. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer. J. Clin. 2010, 60, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Sriparna, G.; Sullivan, C.A.W.; Zerkowski, M.P.; Molinaro, A.M.; Rimm, D.L.; Camp, R.L.; Chung, G.G. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta 2010, 1806, 108–121. [Google Scholar]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 2004; p. 130. [Google Scholar]
- Boonyarathanakornkit, L.; Che, C.T.; Fong, H.H.; Farnsworth, N.R. Constituents of Croton crassifolius roots. Planta Med. 1988, 54, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Li, G.Q.; Li, J.G. Two new clerodane diterpenoids from Croton crassifolius. Heterocycles 2014, 89, 1585–1593. [Google Scholar]
- Huang, W.; Wang, J.; Liang, Y.; Ge, W.; Wang, G.; Li, Y.; Chung, H.Y. Potent anti-angiogenic component in Croton crassifolius and its mechanism of action. J. Ethnopharmacol. 2015, 175, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Chung, H.Y.; Zhang, Y.B.; Li, G.Q.; Li, Y.L.; Huang, W.H.; Wang, G.C. Diterpenoids from the roots of Croton crassifolius and their anti-angiogenic activity. Phytochemistry 2016, 122, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.M.; Wolff, R.A.; Bogaard, K.; Waldrum, S.; Abbruzzese, J.L. A phase I study of escalating doses of the tyrosine kinase inhibitor semaxanib (SU5416) in combination with irinotecan in patients with advanced colorectal carcinoma. Jpn. J. Clin. Oncol. 2006, 36, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Gingras, D.; Lamy, S.; Béliveau, R. Tyrosine phosphorylation of the vascular endothelial-growth-factor receptor-2 (VEGFR-2) is modulated by Rho proteins. Biochem. J. 2000, 348, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Jiang, Y.; Chu, L.; Hao, H.; Liua, Z.; Verfaillie, C.; Zweier, J.; Gupta, K.; Liu, Z. MAPK/ERK signaling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J. Cell. Mol. Med. 2008, 12, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Sanni, S.B.; Behm, H.; Beurskens, P.T.; Adesogan, E.K. Structure of ent-(12R)-methyl-15,16-epoxy-9,10-friedolabda-5(10),13(16),14-trien-19-oate-20,12-lactone (penduliflaworonsin). Acta Crystallogr. 1987, 43, 555–558. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Foubert, K.; Chacha, M.; Kapingu, M.C.; Magadula, J.J.; Moshi, M.M.; Lemiere, F.; Goubitz, K.; Fraanje, J.; Peschar, R.; et al. New furanoditerpenoids from Croton jatrophoides. Planta Med. 2009, 75, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Apoptosis signaling in tumor therapy. Ann. N. Y. Acad. Sci. 2004, 1028, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Li, J.G.; Li, G.Q.; Xu, J.J.; Wu, X.; Ye, W.C.; Li, Y.L. Clerodane Diterpenoids from Croton crassifolius. J. Nat. Prod. 2012, 75, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liang, Y.; Wang, J.; Li, G.; Wang, G.; Li, Y.; Chung, H.Y. Anti-angiogenic activity and mechanism of kaurane diterpenoids from Wedelia chinensis. Phytomedicine 2016, 23, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, S.; Liu, Z.; Zhang, W.; Xu, J.; Wang, Y.; Liu, J.; Zhang, D.; Tian, H.; Li, Y. Arenobufagin, a bufadienolide compound from toad venom, inhibits VEGF-mediated angiogenesis through suppression of VEGFR-2 signaling pathway. Biochem. Pharmacol. 2012, 83, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Zhang, Y.; Wang, G.; Li, Y.; Huang, W. Penduliflaworosin, a Diterpenoid from Croton crassifolius, Exerts Anti-Angiogenic Effect via VEGF Receptor-2 Signaling Pathway. Molecules 2017, 22, 126. https://doi.org/10.3390/molecules22010126
Liang Y, Zhang Y, Wang G, Li Y, Huang W. Penduliflaworosin, a Diterpenoid from Croton crassifolius, Exerts Anti-Angiogenic Effect via VEGF Receptor-2 Signaling Pathway. Molecules. 2017; 22(1):126. https://doi.org/10.3390/molecules22010126
Chicago/Turabian StyleLiang, Yeyin, Yubo Zhang, Guocai Wang, Yaolan Li, and Weihuan Huang. 2017. "Penduliflaworosin, a Diterpenoid from Croton crassifolius, Exerts Anti-Angiogenic Effect via VEGF Receptor-2 Signaling Pathway" Molecules 22, no. 1: 126. https://doi.org/10.3390/molecules22010126




