The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride—In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microparticles Morphology, Size and Surface Charge Analysis
2.2. Swelling and Mucoadhesive Properties
2.3. In Vitro MF Release
2.4. Stability Studies
2.5. Differential Scanning Calorimetry (DSC)
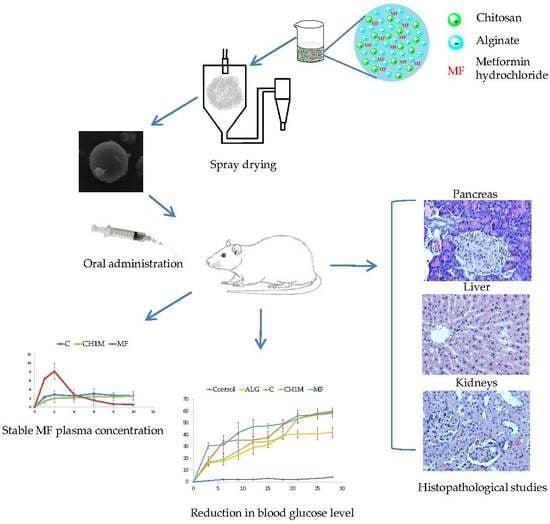
2.6. In Vivo Studies
2.6.1. Blood Glucose Levels
2.6.2. Pharmacokinetic Parameters
2.6.3. Histopathological Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan Cross-Linked ALG Microparticles by Spray Drying
3.3. Characteristics of Microparticles
3.3.1. Morphology and Size
3.3.2. HPLC Analysis
3.3.3. MF Loading and Percentage Yield
3.3.4. Zeta Potential
3.3.5. Swelling Properties
3.3.6. Mucoadhesiveness
3.4. In Vitro MF Release
3.5. Mathematical Modeling of MF Release Profile
3.6. Stability Studies
3.7. Differential Scanning Calorimetry (DSC)
3.8. In Vivo Studies
3.8.1. Animals
3.8.2. Experimental Design
3.8.3. Blood Glucose Levels
3.8.4. MF Pharmacokinetic Parameters
3.8.5. Histopathological Studies
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sachan, K.N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W. Alginate graft copolymers and alginate–co-excipient physical mixture in oral drug delivery. J. Pharm. Pharmacol. 2011, 63, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Jinno, D.; Liu, D.; Isobe, T.; Kofuji, K.; Kawashima, S. The drug release profile from calcium-induced alginate gel beads coated with an alginate hydrolysate. Molecules 2007, 12, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling concept combining chitosan and alginate—Proof of principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, H.; Lin, J.; Xie, W.; Ma, X. Characterization and biodegradation of chitosan–alginate polyelectrolyte complexes. Polym. Degrad. Stab. 2009, 94, 1–6. [Google Scholar] [CrossRef]
- Wittaya-areekul, S.; Kruenate, J.; Prahsarn, C. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int. J. Pharm. 2006, 312, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. Influence of unmodified and β-glycerophosphate cross-linked chitosan on anti-candida activity of clotrimazole in semi-solid delivery systems. Int. J. Mol. Sci. 2014, 15, 17765–17777. [Google Scholar] [CrossRef] [PubMed]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Arianto, A.; Bangun, H.; Harahap, U.; Ilyas, S. Effect of alginate chitosan ratio on the swelling, mucoadhesive, and release of ranitidine from spherical matrices of alginate-chitosan. Int. J. PharmTech Res. 2015, 8, 653–665. [Google Scholar]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulito, C.; Sanli, T.; Rana, P.; Muti, P.; Blandino, G.; Strano, S. Metformin: On ongoing journey across diabetes, cancer therapy and prevention. Metabolites 2013, 3, 1051–1075. [Google Scholar] [CrossRef] [PubMed]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mythri, G.; Kavitha, K.; Kumar, M.R.; Jagadeesh Singh, S.D. Novel mucoadhesive polymers—A review. J. Appl. Pharm. Sci. 2011, 1, 37–42. [Google Scholar]
- Zhang, X.; Tian, F.; Hou, Y.; Ou, Z. Preparation and in vitro in vivo characterization of polyelectrolyte alginate–chitosan complex based microspheres loaded with verapamil hydrochloride for improved oral drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 429–440. [Google Scholar] [CrossRef]
- Jia, M.; Li, Z.; Chu, H.; Li, L.; Chen, K. Alginate-chitosan microspheres for controlled drug delivery of diltiazem hydrochloride in cardiac diseases. J. Biomater. Tissue Eng. 2015, 5, 246–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and evaluation of alginate-chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Wróblewska, M.; Sosnowska, K.; Winnicka, K. Influence of sodium alginate on hypoglycemic activity of metformin hydrochloride in the microspheres obtained by the spray drying. Int. J. Polym. Sci. 2016, 2016, 8635408. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [PubMed]
- Pestov, A.; Bratskaya, S. Chitosan and its derivatives as highly efficient polymer ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.A.; Smart, J.D. An investigation into the role of water movement and mucus gel dehydration in mucoadhesion. J. Control. Release 1993, 25, 197–203. [Google Scholar] [CrossRef]
- Haugstad, K.E.; Håti, A.G.; Nordgård, C.T.; Adl, P.S.; Maurstad, G.; Sletmoen, M.; Draget, K.I.; Dias, R.S.; Stokke, B.T. Direct determination of chitosan–mucin interactions using a single-molecule strategy: Comparison to alginate–mucin interactions. Polymers 2015, 7, 161–185. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, K. Polymers in mucoadhesive drug-delivery systems: A brief note. Design Monomer Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Jackson, S.J.; Perkins, A.C. In vitro assessment of the mucoadhesion of cholestyramine to porcine and human gastric mucosa. Eur. J. Pharm. Biopharm. 2001, 52, 121–127. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gupta, A.B.; Kumar, R.; Yadav, J.S.; Kumar, B. Mucoadhesive polymers: Means of improving the mucoadhesive properties of drug delivery system. J. Chem. Pharm. Res. 2010, 2, 418–432. [Google Scholar]
- Panizzon, G.P.; Bueno, F.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Preparation of spray-dried soy isoflavone-loaded gelatin microspheres for enhancement of dissolution: Formulation, characterization and in vitro evaluation. Pharmaceutics 2014, 6, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatin. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [PubMed]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quím. 2004, 29, 53–56. [Google Scholar] [CrossRef]
- Mucha, M.; Pawlak, A. Thermal analysis of chitosan and its blends. Thermochim. Acta 2005, 427, 69–76. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.Y.; Li, Y.; Xu, Z.G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.G.; Kristensen, M.; Astrup, A. Effect of alginate supplementation on weight loss in obese subjects completing a 12-wk energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, N. Sodium alginate—A potential tool for weight management: Effect on subjective appetite, food intake, and glycemic and insulin regulation. J. Undergard. Life Sci. 2012, 6, 66–69. [Google Scholar]
- Deshpande, A.; Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.K.; Dusseault, J.; Bilodeau, S.; Langlois, G.; Hallé, J.P.; Yahia, L. Factors influencing alginate gel biocompatibility. J. Biomed. Mater. Res. A 2011, 98, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Nugent, D.A.; Smith, D.M.; Jones, H.B. A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol. Pathol. 2008, 36, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Robles, N.R.; Villa, J.; Gallego, R.H. Non-proteinuric diabetic nephropathy. J. Clin. Med. 2015, 4, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination degree of deacetylation of chitosan: Comparison of methods. Prog. Chem. Appl. Chitin Deriv. 2012, 17, 5–20. [Google Scholar]
- Szekalska, M.; Winnicka, K.; Czajkowska-Kośnik, A.; Sosnowska, K.; Amelian, A. Evaluation of alginate microspheres with metronidazole obtained by the spray drying technique. Acta Pol. Pharm. Drug Res. 2015, 72, 569–578. [Google Scholar]
- The European Pharmacopeia, 6th ed.; Council of Europe: Strasburg, France, 2007; Volume 2, pp. 1490–1491.
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Mazurek-Wądołkowska, E.; Winnicka, K.; Czajkowska-Kośnik, A.; Czyżewska, U.; Miltyk, W. Application of differential scanning calorimetry in evaluation of solid state interactions in tablets containing acetaminophen. Acta Pol. Pharm. Drug Res. 2013, 70, 787–793. [Google Scholar]
- Chhetri, H.P.; Thapa, P.; Van Schepdael, A. Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation. Saudi Pharm. J. 2014, 22, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wróblewska, M.; Sosnowska, K.; Car, H.; Kasacka, I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des. Dev. Ther. 2015, 9, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Kasacka, I.; Janiuk, I.; Lewandowska, A.; Bekisz, A.; Lebkowski, W. Distribution pattern of CART-containing neurons and cells in the human pancreas. Acta Histochem. 2012, 114, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.









| Formulation | Zeta Potential (mV) | Production Yield (%) | Encapsulation Efficiency (%) | Percent Loading (%) | Mean Diameter (µm) | Polydispersity Index |
|---|---|---|---|---|---|---|
| C | −1.3 ± 0.7 | 61.7 ± 2.1 | 113.4 ± 2.3 | 75.6 ± 1.5 | 3.0 ± 1.6 | 0.38 ± 0.1 |
| CH1M | 6.7 ± 1.3 | 61.8 ± 1.5 | 94.1 ± 3.2 | 79.9 ± 1.6 | 2.3 ± 1.1 | 0.52 ± 0.2 |
| CH2M | 5.2 ± 0.3 | 85.9 ± 1.4 | 95.4 ± 1.9 | 76.5 ± 2.2 | 3.2 ± 1.7 | 0.42 ± 0.2 |
| Formulation | Type of Adhesive Layer | |||||
|---|---|---|---|---|---|---|
| Gelatin | Mucin | Porcine Stomach Mucosa | ||||
| Fmax (N) * | Wad (µJ) ** | Fmax (N) * | Wad (µJ) ** | Fmax (N) * | Wad (µJ) ** | |
| C | 0.5 ± 0.2 | 283.3 ± 50.6 | 0.6 ± 0.3 | 342.3 ± 29.1 | 0.6 ± 0.2 | 467.5 ± 17.9 |
| CH1P | 0.5 ± 0.1 | 314.8 ± 13.9 | 0.8 ± 0.2 | 384.7 ± 29.7 | 1.4 ± 0.2 | 782.6 ± 29.2 |
| CH2P | 0.5 ± 0.2 | 312.4 ± 21.2 | 0.7 ± 0.2 | 381.2 ± 13.1 | 1.3 ± 0.2 | 564.4 ± 18.6 |
| CH1M | 0.5 ± 0.1 | 310.1 ± 16.8 | 0.8 ± 0.3 | 378.7 ± 35.5 | 1.2 ± 0.1 | 779.5 ± 24.8 |
| CH2M | 0.6 ± 0.2 | 293.6 ± 40.4 | 0.7 ± 0.1 | 349.4 ± 31.3 | 1.2 ± 0.2 | 549.1 ± 23.7 |
| Formulation | Zero Order Kinetics | First Order Kinetics | Highuchi Model | Korsmeyer–Peppas Model | Hixson–Crowell Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | n | R2 | K | |
| C | 0.52 | 4.62 | 0.73 | 0.21 | 0.66 | 18.76 | 0.59 | 0.28 | 0.12 | 0.65 | 0.19 |
| CH1M | 0.71 | 6.27 | 0.97 | 0.18 | 0.85 | 31.55 | 0.76 | 0.27 | 0.11 | 0.93 | 0.57 |
| CH2M | 0.87 | 6.69 | 0.95 | 0.22 | 0.96 | 25.03 | 0.90 | 0.32 | 0.19 | 0.94 | 0.22 |
| Evaluation Parameter | Formulation | |||
|---|---|---|---|---|
| C | CH1M | CH2M | ||
| Mean diameter (µm) | 2.7 ± 1.3 | 2.1 ± 1.5 | 2.9 ± 1.4 | |
| Percent drug loading (%) | 73.1 ± 5.7 | 73.6 ± 4.3 | 70.7 ± 1.4 | |
| Zeta potential (mV) | −1.16 ± 0.3 | 5.9 ± 1.4 | 4.8 ± 0.6 | |
| Mucoadhesion to: | ||||
| Gelatin disc | Fmax (N) * | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Wad (µJ) ** | 288.2 ± 18.8 | 304.8 ± 26.8 | 233.6 ± 19.3 | |
| Mucin gel | Fmax (N) * | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.2 |
| Wad (µJ) ** | 316.8 ± 17.9 | 318.6 ± 21.7 | 323.2 ± 26.7 | |
| Porcine stomach mucosa | Fmax (N) * | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| Wad (µJ) ** | 379.7 ± 36.2 | 715.9 ± 31.6 | 456.1 ± 34.6 | |
| Formulation | Cmax (μg/mL) | tmax (h) | AUC0→10 (μg·h/mL) |
|---|---|---|---|
| C | 3.03 ± 0.70 | 6.0 | 30.51 ± 0.51 |
| CH1M | 2.37 ± 0.34 | 6.0 | 22.10 ± 0.38 |
| MF | 8.13 ± 0.50 | 2.0 | 23.43 ± 0.78 |
| Formulation | Concentration of ALG Solution (w/w) | Concentration of Chitosan Solution (w/w) | MF:ALG Ratio |
|---|---|---|---|
| C | 2% | - | 2:1 |
| CH1P | 2% | 0.10 | - |
| CH2P | 2% | 0.05 | - |
| CH1M | 2% | 0.10 | 2:1 |
| CH2M | 2% | 0.05 | 2:1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekalska, M.; Sosnowska, K.; Zakrzeska, A.; Kasacka, I.; Lewandowska, A.; Winnicka, K. The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride—In Vitro and In Vivo Evaluation. Molecules 2017, 22, 182. https://doi.org/10.3390/molecules22010182
Szekalska M, Sosnowska K, Zakrzeska A, Kasacka I, Lewandowska A, Winnicka K. The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride—In Vitro and In Vivo Evaluation. Molecules. 2017; 22(1):182. https://doi.org/10.3390/molecules22010182
Chicago/Turabian StyleSzekalska, Marta, Katarzyna Sosnowska, Agnieszka Zakrzeska, Irena Kasacka, Alicja Lewandowska, and Katarzyna Winnicka. 2017. "The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride—In Vitro and In Vivo Evaluation" Molecules 22, no. 1: 182. https://doi.org/10.3390/molecules22010182