Achiral Molecular Recognition of Aromatic Position Isomers by Polysaccharide-Based CSPs in Relation to Chiral Recognition
Abstract
:1. Introduction
2. Result and Discussion
2.1. Polysaccharide-Based CSPs
2.2. Separation of Some Sets of Aromatic Position Isomers
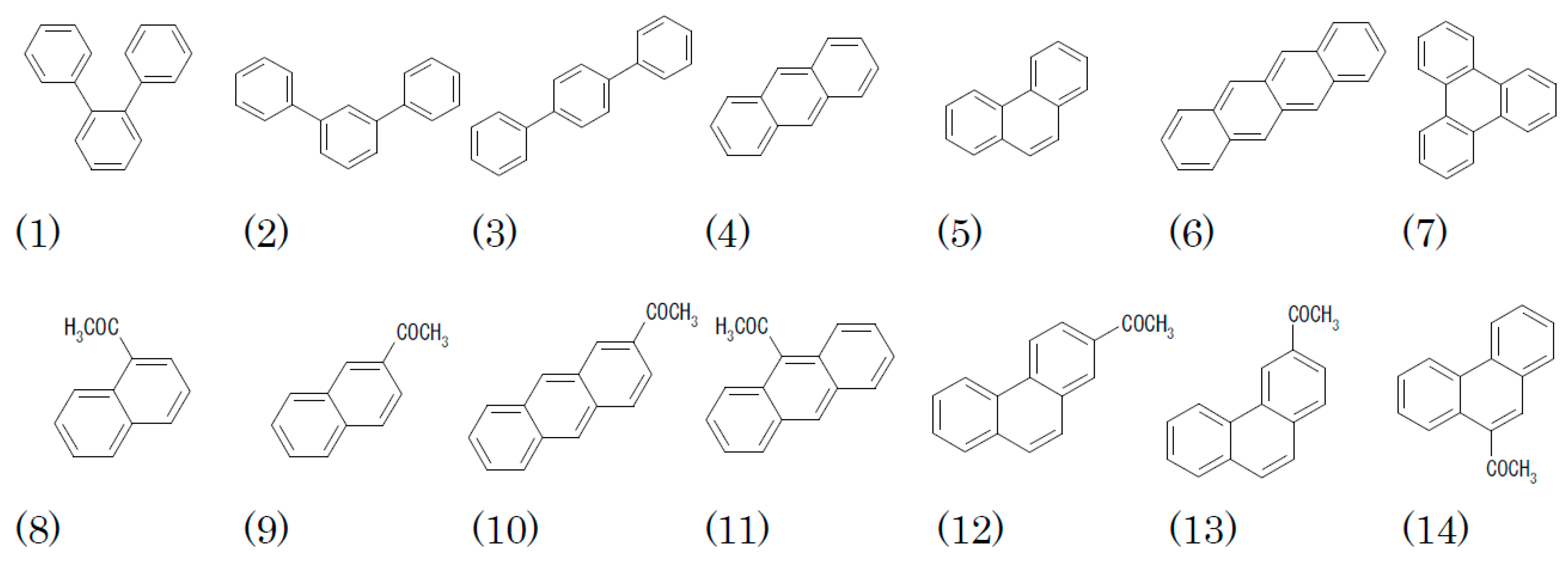
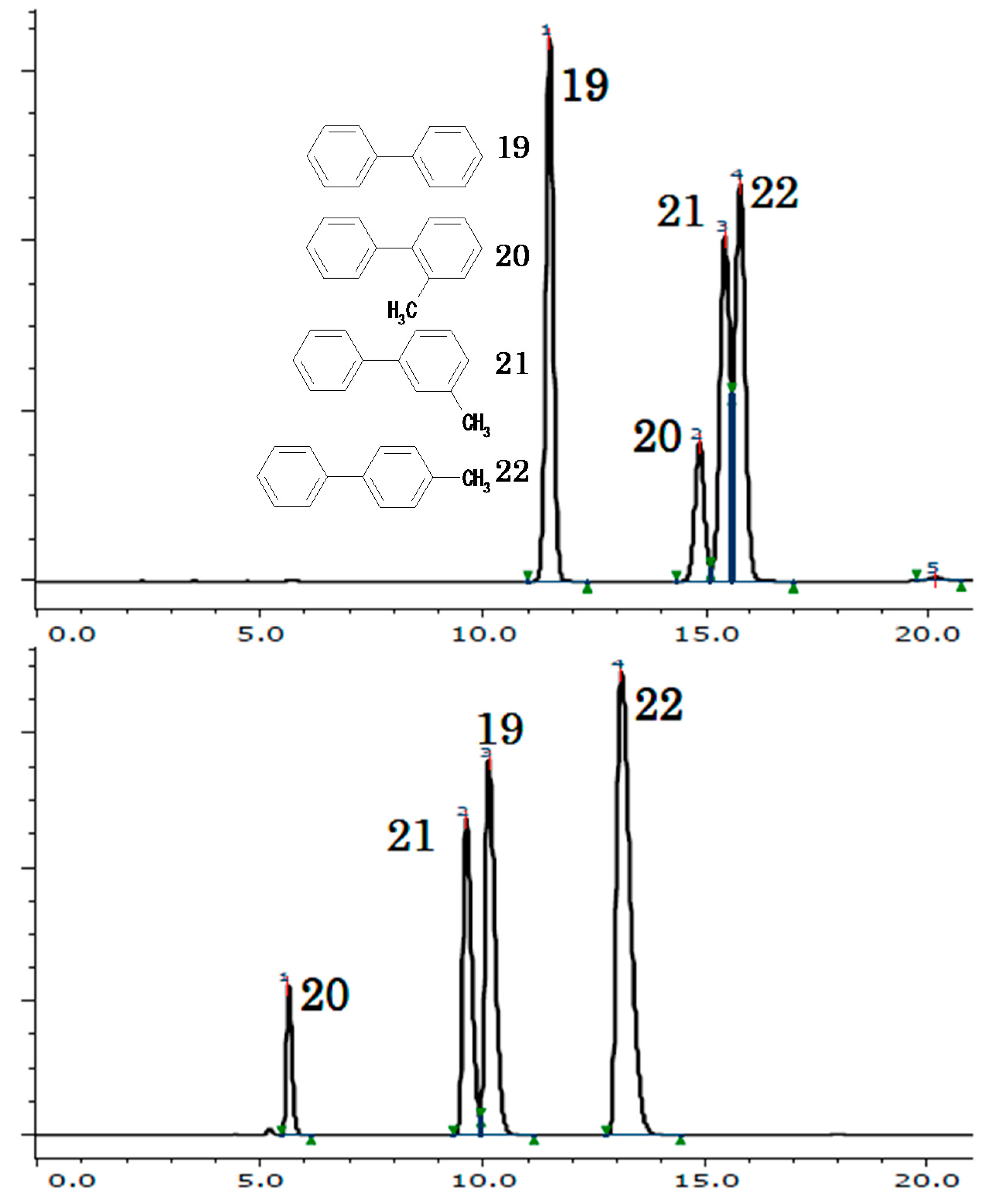
2.2.1. Terphenyls and Triphenylene
2.2.2. Polycondensed Aromatic Hydrocarbons (PAHs)
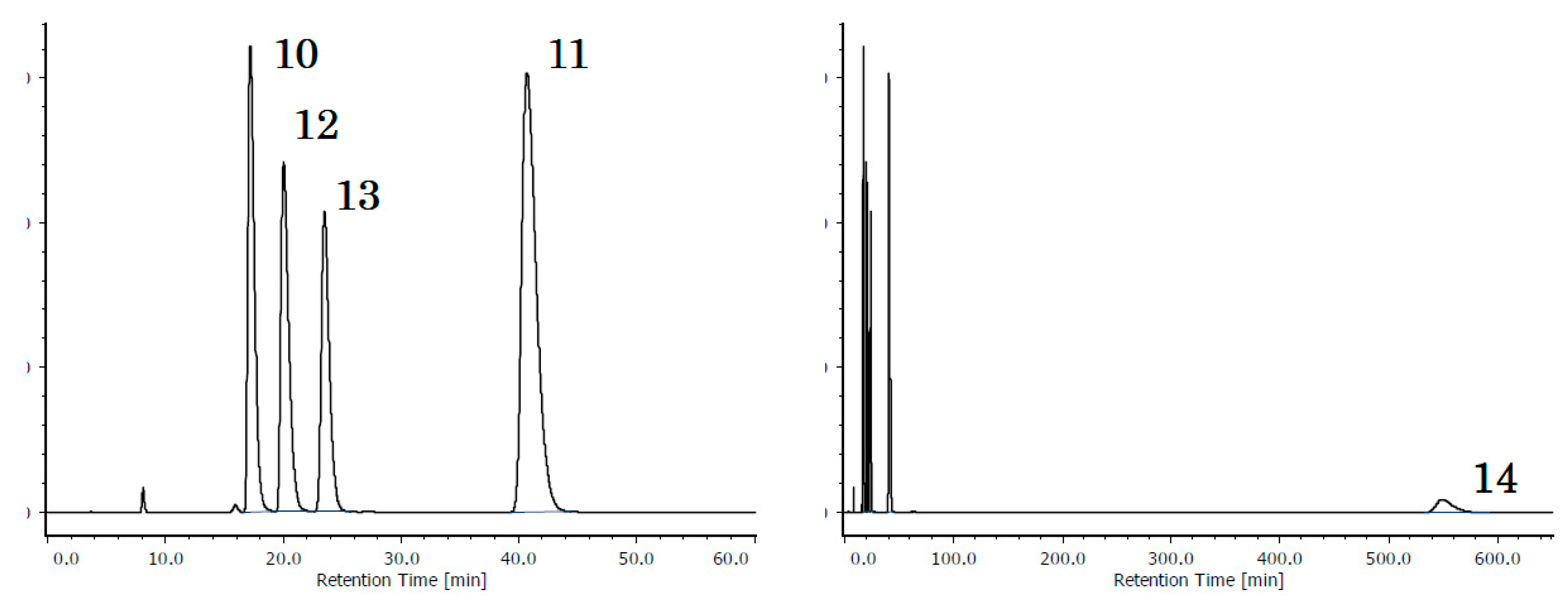
2.2.3. Acetyl PAHs
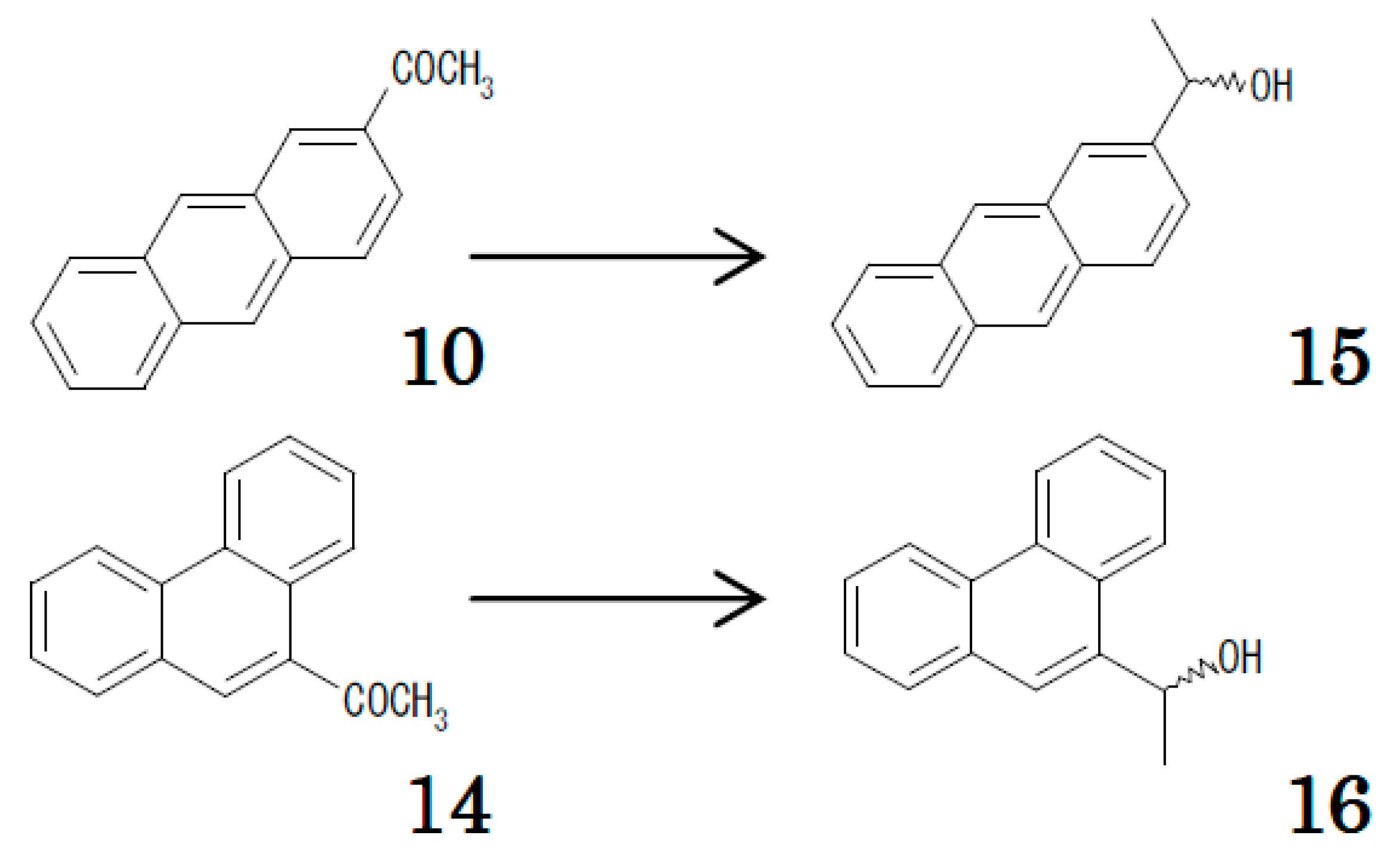
2.3. Relation between Chiral and Achiral Recognition
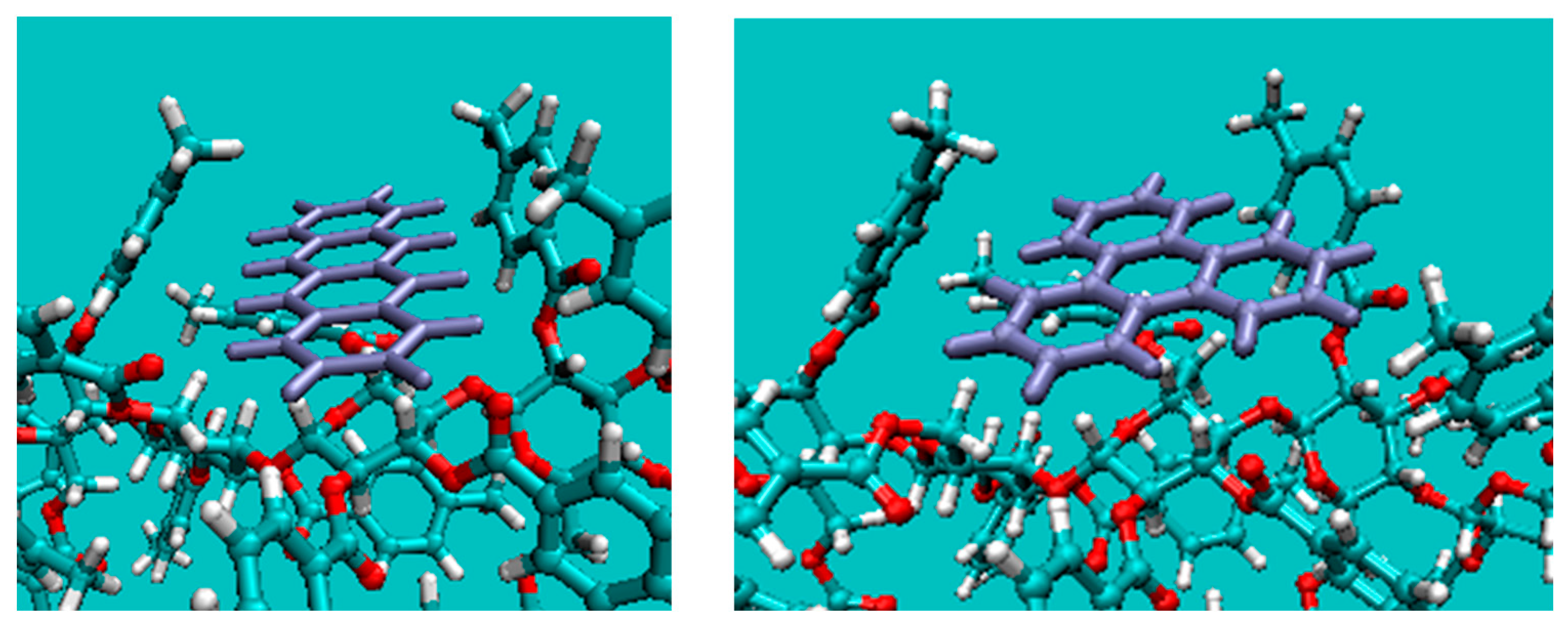
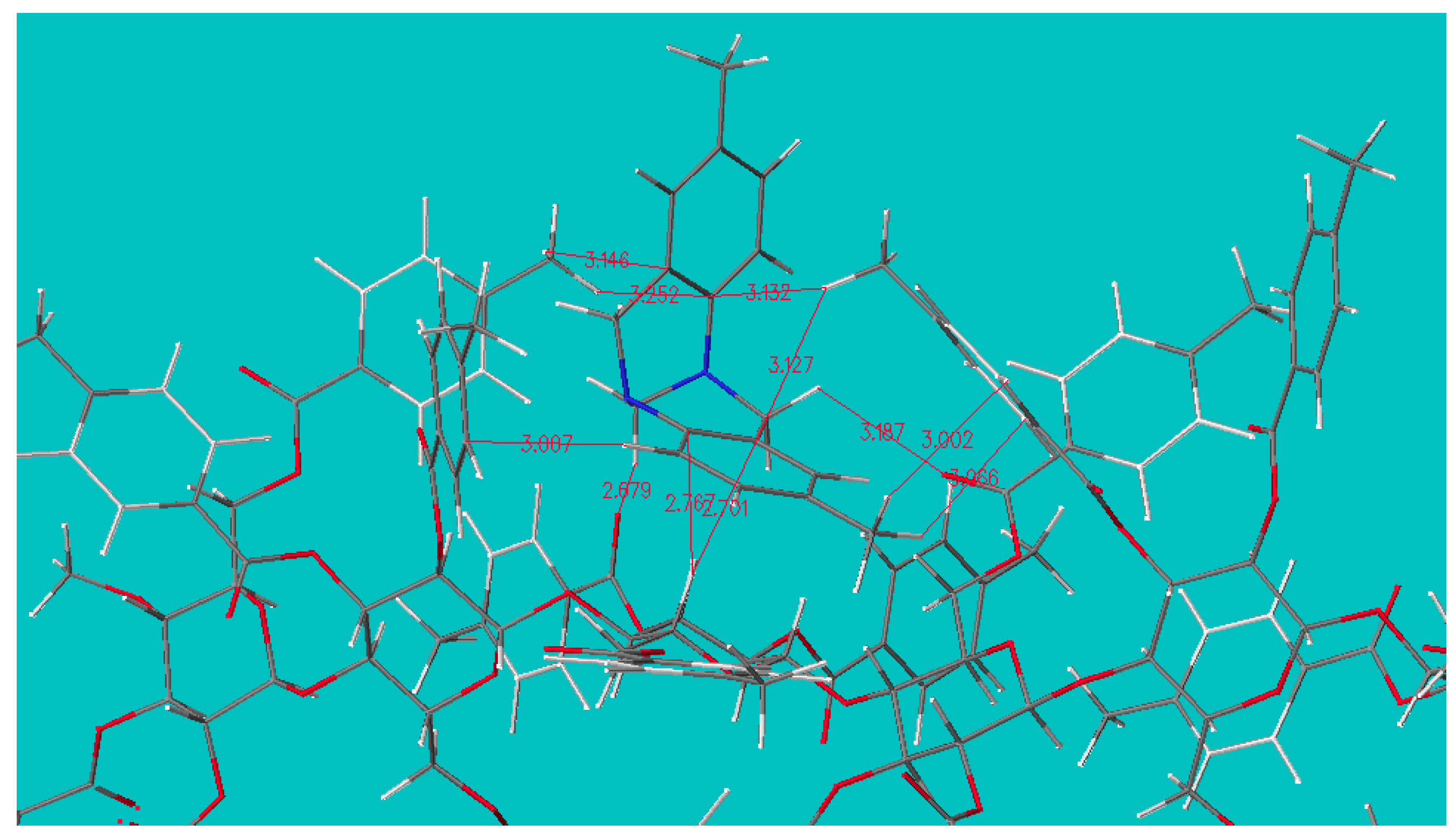
2.4. Origin of the Selectivity
2.4.1. Conformer Contributing Molecular Recognition
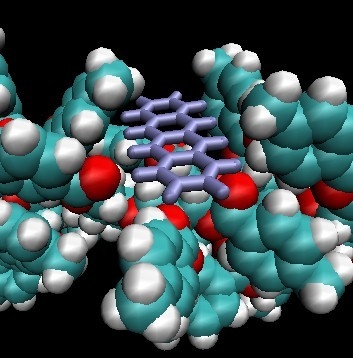
2.4.2. Docking Simulation
2.5. Difference between ODS Phase and Polysaccharide-Based CSPs
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Chromatographic Conditions
3.3. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okamoto, Y.; Yashima, E. Polysaccharide derivatives for chromatographic separation of enantiomers. Angew. Chem. Int. Ed. 1998, 37, 1020–1043. [Google Scholar] [CrossRef]
- Ikai, T.; Okamoto, Y. Chiral HPLC for efficient resolution of enantiomers. Chem. Rev. 2009, 109, 6077–6101. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Okamoto, Y.; Ishii, K. Chromatographic optical resolution on polysaccharides and their derivatives. J. Liq. Chromatogr. 1986, 9, 313–340. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y. High-performance liquid chromatographic enantioseparation of drugs containing multiple chiral centers on polysaccharide-type chiral stationary phases. J. Chromatogr. A 2001, 906, 185–193. [Google Scholar] [CrossRef]
- Kanazawa, H.; Tsubayashi, A.; Nagata, Y.; Matsushima, Y.; Mori, C.; Kizu, J.; Higaki, M. Stereospecific analysis of loxoprofen in plasma by chiral column liquid chromatography with a circular dichroism-based detector. J. Chromatogr. A 2002, 948, 303–308. [Google Scholar] [CrossRef]
- Mesplet, N.; Saito, Y.; Morin, P.; Agrofoglio, L.A. Liquid chromatographic separation of phosphoramidate diastereomers on a polysaccharide-type chiral stationary phase. J. Chromatogr. A 2003, 983, 115–124. [Google Scholar] [CrossRef]
- Lipka-Belloli, E.; Len, C.; Mackenzie, G.; Ronco, G.; Bontea, J.-P.; Vacchera, C. Diastereomeric resolution of nucleoside analogues, new potential antiviral agents, using high-performance liquid chromatography on polysaccharide-type chiral stationary phases. J. Chromatogr. A 2002, 943, 91–100. [Google Scholar] [CrossRef]
- Lipka, E.; Selouane, A.; Postel, D.; Lenb, C.; Vaccher, M.P.; Bonte, J.-P.; Vaccher, C. Enantioseparation of four cis and trans diastereomers of 2,3-didehydro-2,3-dideoxythymidine analogs, by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2004, 1034, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; la Torre, F.; Mai, A.; Rotili, D. Analytical and semipreparative high performance liquid chromatography separation of stereoisomers of novel 3,4-dihydropyrimidin-4(3H)-one derivatives on the immobilised amylose-based Chiralpak IA chiral stationary phase. J. Sep. Sci. 2006, 29, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, H.; Fu, Q.-M.; Li, Z.-Y. The Chiral Pyrethroid Cycloprothrin: Stereoisomer Synthesis and Separation and Stereoselective Insecticidal Activity. Chirality 2008, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Sellers, J.A.; Bernard, A.; Olsen, B.A.; Owens, P.K.; Gavin, P.F. Determination of the enantiomer and positional isomer impurities in atomoxetine hydrochloride with liquid chromatography using polysaccharide chiral stationary phases. J. Pharm. Biomed. Anal. 2006, 41, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Mizobe, H.; Otake, I.; Ichioka, K.; Kojima, K.; Matsumoto, Y.; Gotoh, N.; Kuroda, I.; Wada, S. Enantiomeric separation of asymmetric triacylglycerol by recycle high-performance liquid chromatography with chiral column. J. Chromatogr. A 2011, 1218, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.H. Comparison of chiral separations on polysaccharide chiral stationary phases to an improved Pirkle phase. J. Chromatogr. A 1996, 725, 219–224. [Google Scholar] [CrossRef]
- Cirilli, R.; Ferretti, R.; la Torre, F.; Secci, D.; Bolasco, A.; Carradori, S.; Pierini, M. High-performance liquid chromatographic separation of enantiomers and diastereomers of 2-methylcyclohexanone thiosemicarbazone, and determination of absolute configuration and configurational stability. J. Chromatogr. A 2007, 1172, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Caccamesse, S.; Manna, L.; Scivoli, G. Chiral HPLC Separation and CD Spectra of the C-2Diastereomers of Naringin in Grapefruit during Maturation. Chirality 2003, 15, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pritts, W.A.; Zhang, S. Chiral separation of selected proline derivatives using a polysaccharide type stationary phase by supercritical fluid chromatography and comparison with high-performance liquid chromatography. J. Chromatogr. A 2008, 1189, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Musson, D.G. Reversed-phase chiral liquid chromatography on polysaccharide-based stationary phase coupled with tandem mass spectrometry for simultaneous determination of four stereoisomers of MK-0974 in human plasma. J. Chromatogr. B 2008, 873, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Regalado, E.L.; Welch, C.J. Separation of achiral analytes using supercritical fluid chromatography with chiral stationary phases. Trends Anal. Chem. 2015, 67, 74–81. [Google Scholar] [CrossRef]
- Achiral Separations on Daicel CSPs. Available online: https://www.daicelchiral.com/en.html (accessed on 15 November 2016).
- Yashima, E.Y.; Yamamoto, C.; Okamoto, Y. NMR studies of chiral discrimination relevant to the liquid chromatographic enantioseparation by a cellulose phenylcarbamate derivative. J. Am. Chem. Soc. 1996, 118, 4036–4048. [Google Scholar] [CrossRef]
- Yamamoto, C.; Yamada, K.; Motoya, K.; Kamiya, Y.; Kamigaito, M.; Okamoto, Y.; Aratani, T. Preparation of HPLC chiral packing materials using cellulose tris(4-methylbenzoate) for the separation of chrysanthemate isomers. J. Polym. Sci. A Polym. Chem. 2006, 44, 5087–5097. [Google Scholar] [CrossRef]
- Zhanga, A.; Lai, W.; Suna, J.; Hub, G.; Liuc, W. Probing the chiral separation mechanism and the absolute configuration of malathion, malaoxon and isomalathion enantiomers by chiral high performance liquid chromatography coupled with chiral detector–binding energy computations. J. Chromatogr. A 2013, 1281, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Shibata, T.; Ueda, K. Computational studies on naphthacene and triphenylene discrimination mechanism of cellulose tris(4-methylbenzoate) and cellulose benzoate. Carbohydr. Res. Under review.
- Zugenmaier, P. Crystalline Cellulose and Cellulose Derivatives; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Van den Bussche, S.; Díaz, D.; Fernández-Alonso, M.C.; Pan, W.; Vincent, S.P.; Cuevas, G.; Cañada, F.J.; Jiménez-Barbero, J.; Bartik, K. Aromatic-carbohydrate interactions: An NMR and computational study of model systems. Chem. Eur. J. 2008, 14, 7570–7578. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T. Chiral separation of Trӧger’s base and related racemates on polysaccharide-based CSPs. Unpublished work.
- Ishikawa, A.; Shibata, T. Cellulosic chiral stationary phase under reversed-phase condition. J. Liq. Chromatogr. 1993, 16, 859–878. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are partially available from the authors.

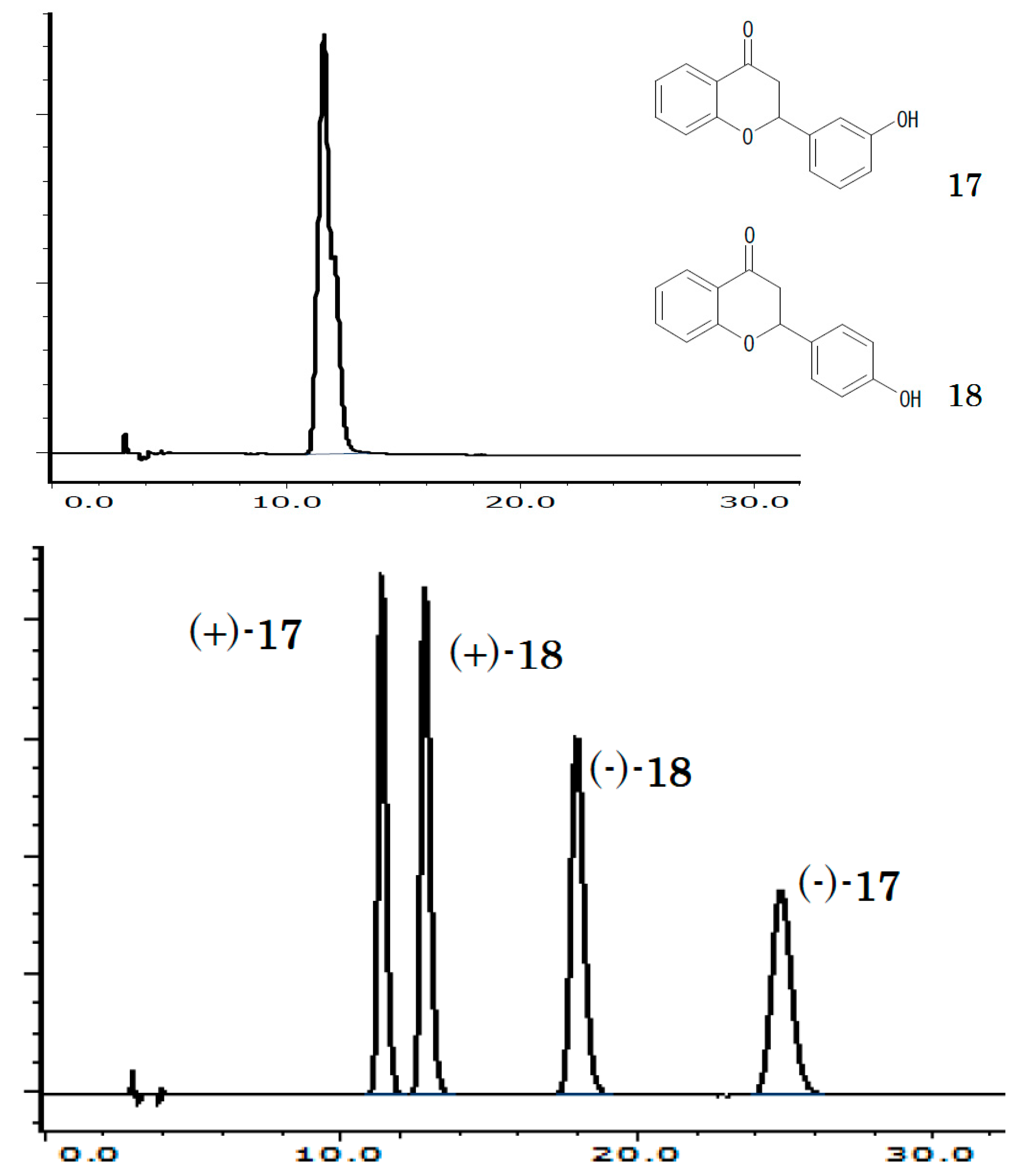
| CSP * | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| “OB-H” | 0.35 | 3.52 | 1.35 | 1.18 | 1.16 | 7.00 | 2.01 |
| “OJ-H” | 0.51 | 1.76 | 4.23 | 6.96 | 5.29 | 52.6 | 3.99 |
| “OD-H” | 0.29 | 0.47 | 0.52 | 0.69 | 1.30 | 1.93 | 28.45 |
| “AD-H” | 0.18 | 0.51 | 0.58 | 0.43 | 0.41 | 2.08 | 0.86 |
| “ODS” | 8.42 | 10.26 | 11.45 | 6.79 | 6.14 | 15.72 | 11.30 |
| CSP | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|
| “OB-H” | 5.33 | 6.7 | 32.98 | 5.94 | 20.19 | 16.64 | 8.03 |
| “OJ-H” | 5.02 | 7.83 | 95.59 | 6.43 | 52.05 | 14.25 | 8.62 |
| “OD-H” | 3.67 | 3.30 | 4.79 | 12.78 | 5.77 | 6.86 | 186.26 |
| “AD-H” | 4.57 | 5.56 | 7.62 | 5.12 | 7.09 | 6.46 | 9.28 |
| “ODS” | 1.67 | 1.66 | 3.61 | 3.25 | 3.41 | 3.33 | 3.24 |
| CSP | Analyte | |||
|---|---|---|---|---|
| 10 | 15 Enantiomers | 14 | 16 Enantiomers | |
| “OJ-H” | 96 | 32.4, 95.2 (α = 2.94) | 8.6 | 4.35, 4.68 (α = 1.07) |
| “OD-H” | 4.8 | 2.42, 4.87 (α = 2.01) | 186 | 20.8, 123 (α = 5.88) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, T.; Shinkura, S.; Ohnishi, A.; Ueda, K. Achiral Molecular Recognition of Aromatic Position Isomers by Polysaccharide-Based CSPs in Relation to Chiral Recognition. Molecules 2017, 22, 38. https://doi.org/10.3390/molecules22010038
Shibata T, Shinkura S, Ohnishi A, Ueda K. Achiral Molecular Recognition of Aromatic Position Isomers by Polysaccharide-Based CSPs in Relation to Chiral Recognition. Molecules. 2017; 22(1):38. https://doi.org/10.3390/molecules22010038
Chicago/Turabian StyleShibata, Tohru, Satoshi Shinkura, Atsushi Ohnishi, and Kazuyoshi Ueda. 2017. "Achiral Molecular Recognition of Aromatic Position Isomers by Polysaccharide-Based CSPs in Relation to Chiral Recognition" Molecules 22, no. 1: 38. https://doi.org/10.3390/molecules22010038