A Novel Role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma
Abstract
:1. Introduction
2. Results
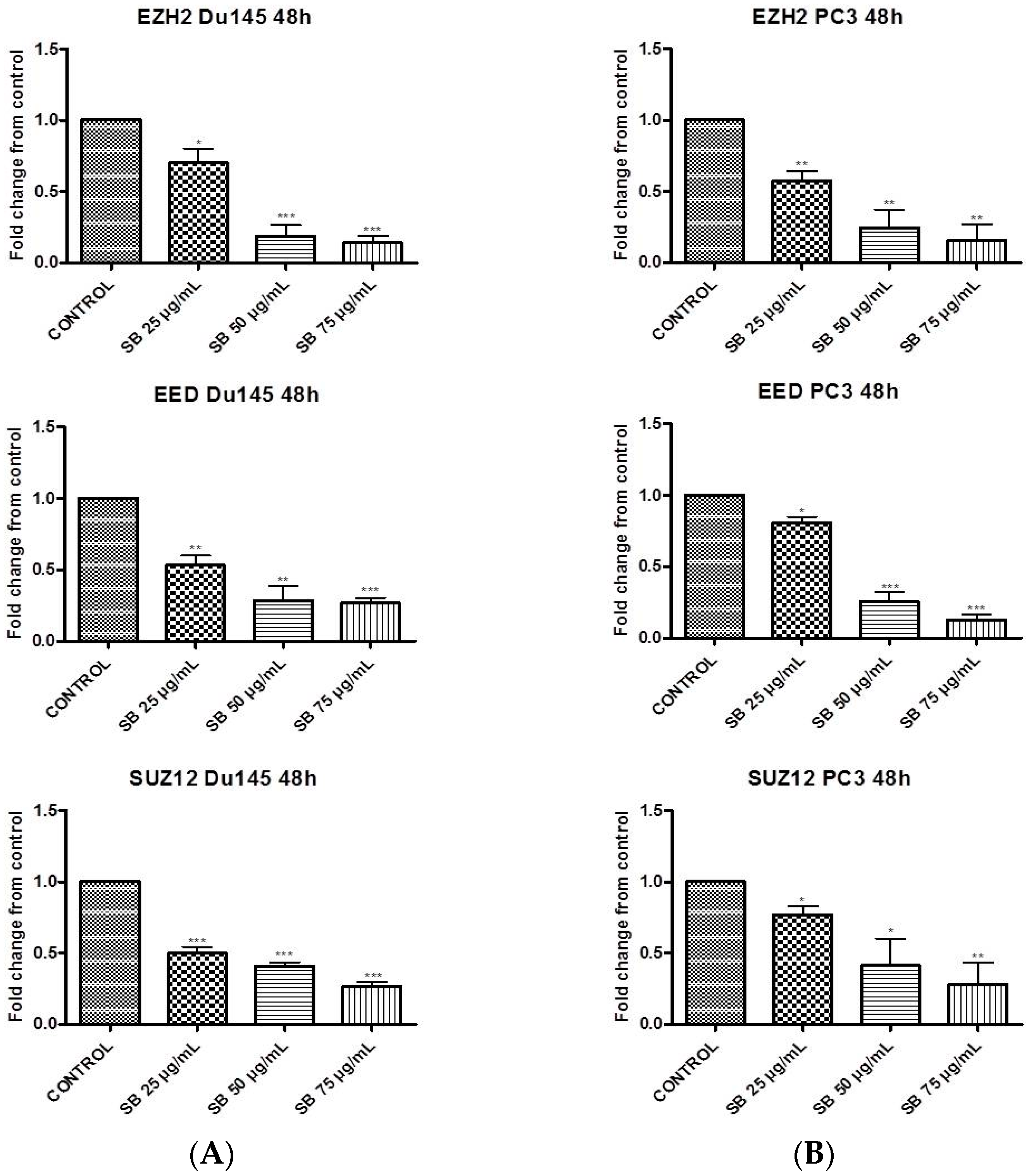
2.1. Silibinin Suppresses PRC2 Complex Members’ Expression
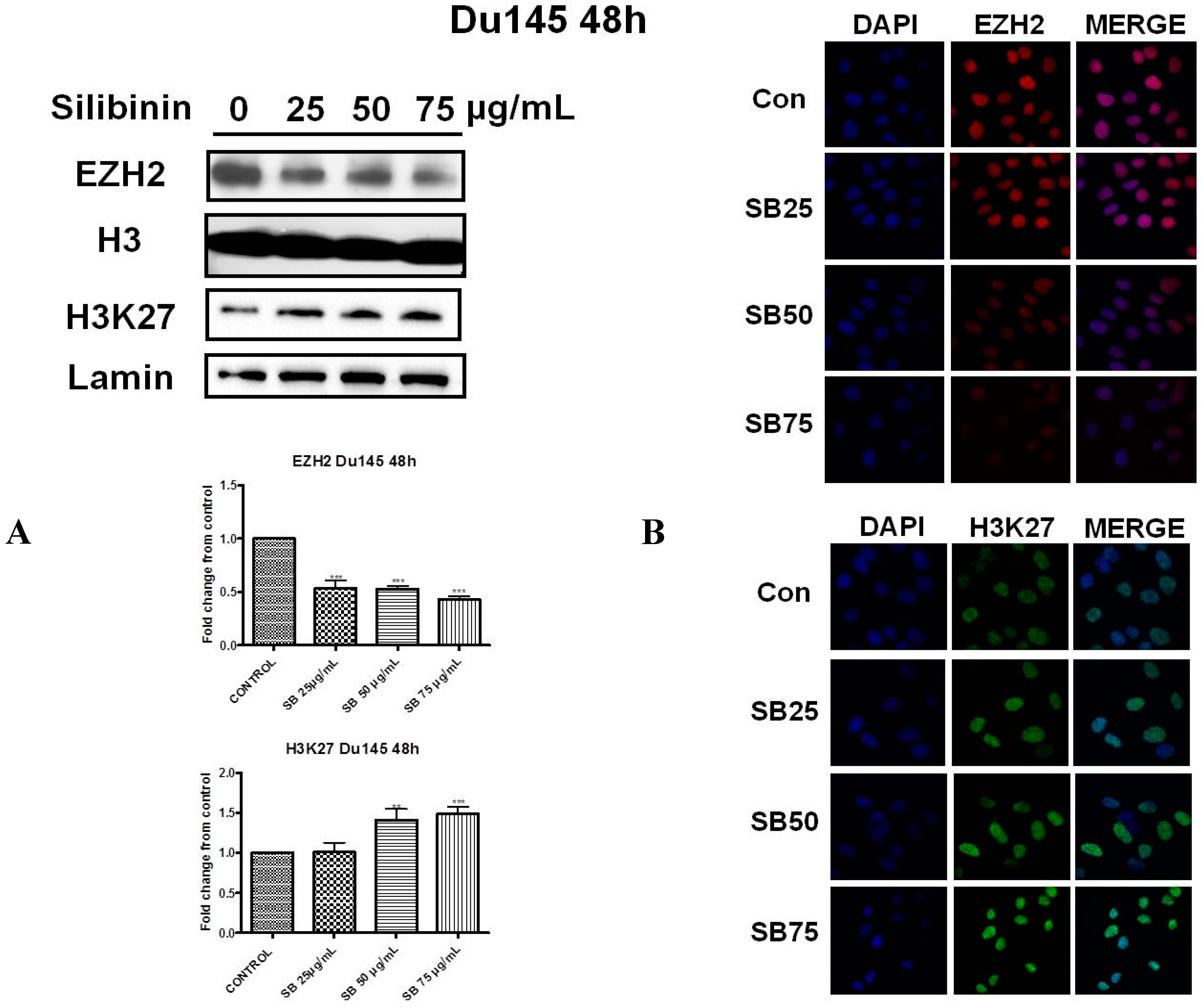
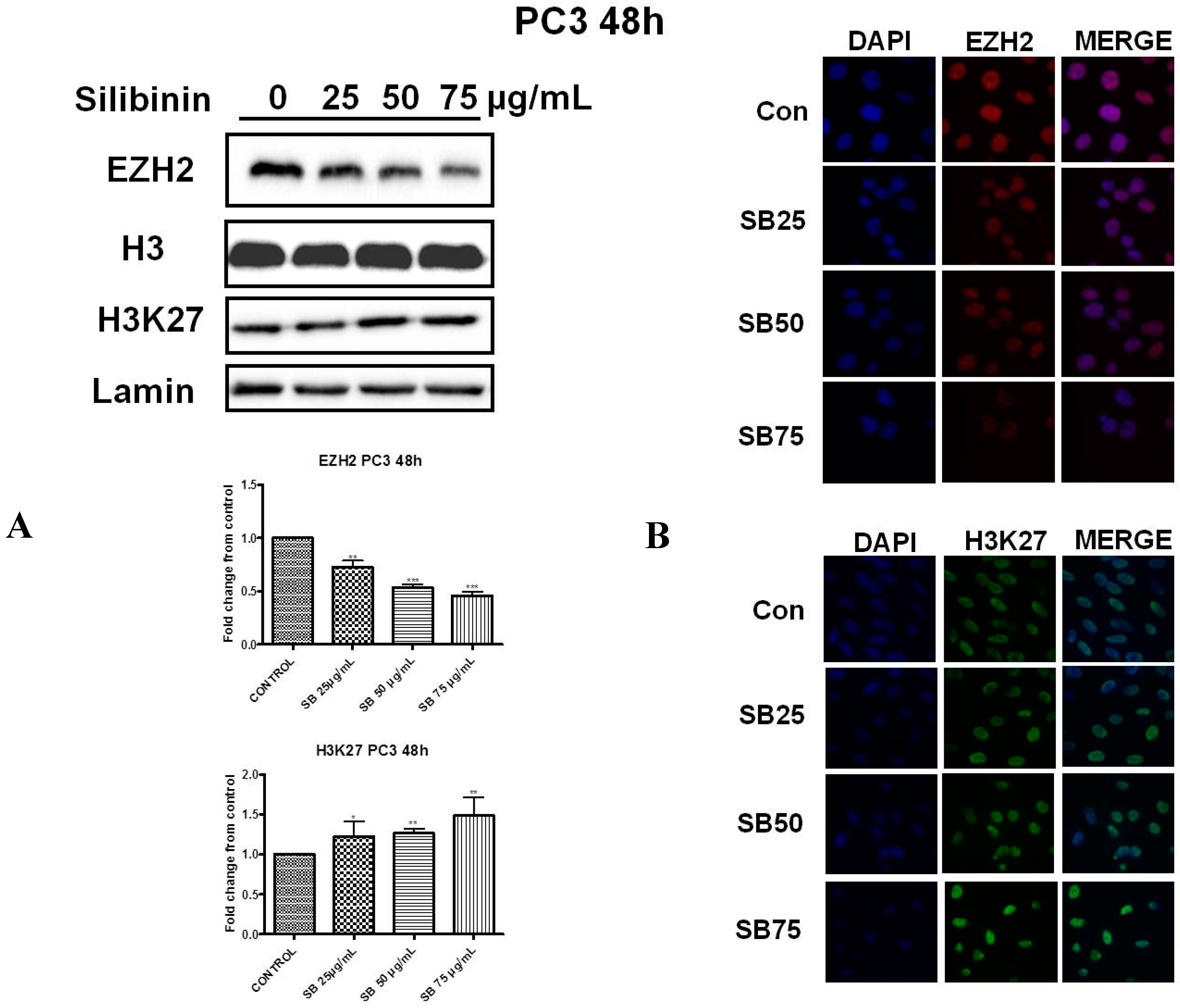
2.2. Silibinin Reduces EZH2 Expression While It Increases Trimethylation Levels of Lys27 on H3 (H3K27me3)
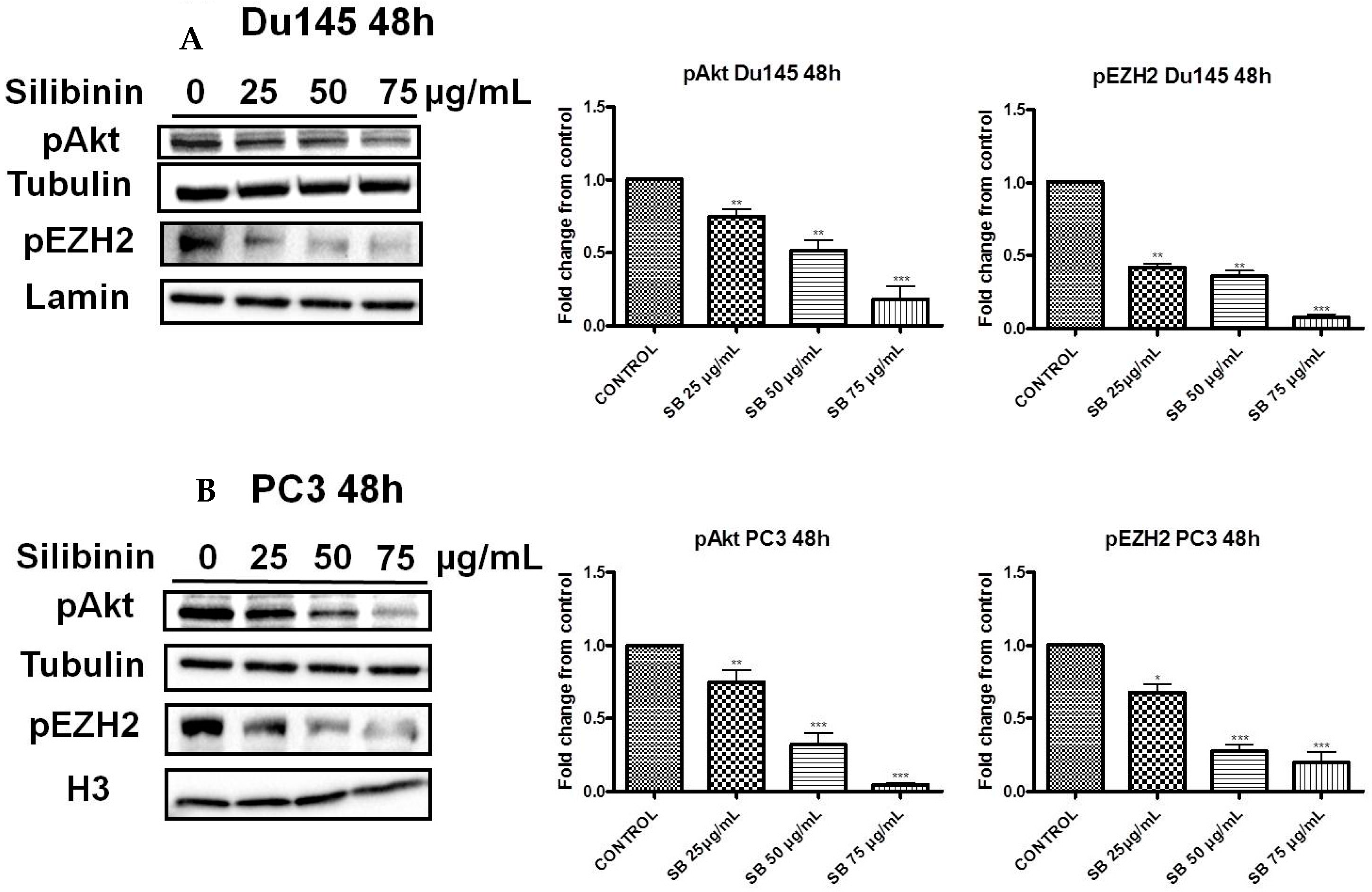
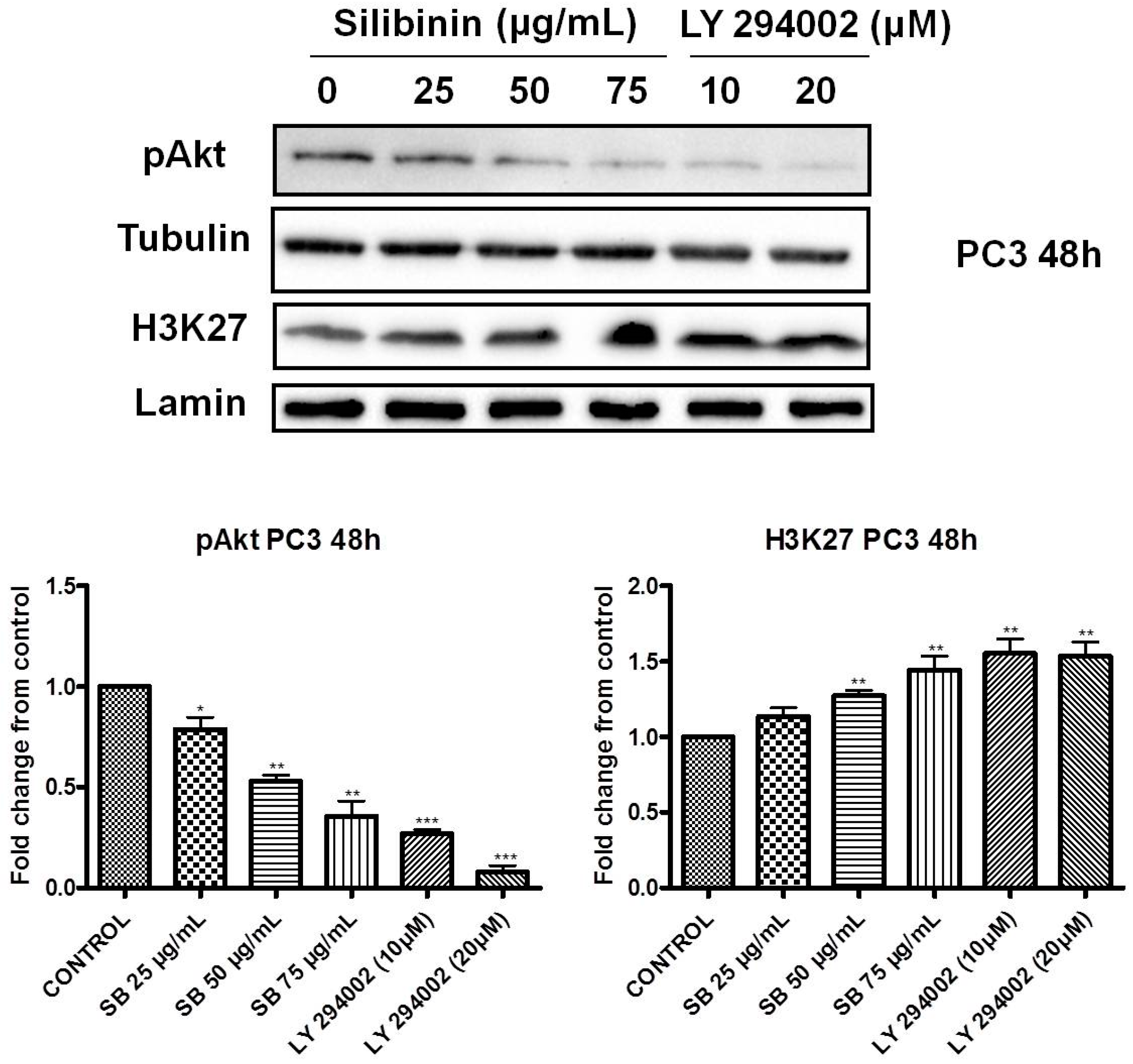
2.3. Silibinin Decreases Phosphorylation of pAkt (ser473) with a Concomitant Suppression of pEZH2 (ser21) Expression Levels
2.4. Increased Trimethylation of Lysine 27 on Histone H3 by Silibinin Is Associated with Decreased pAkt Levels
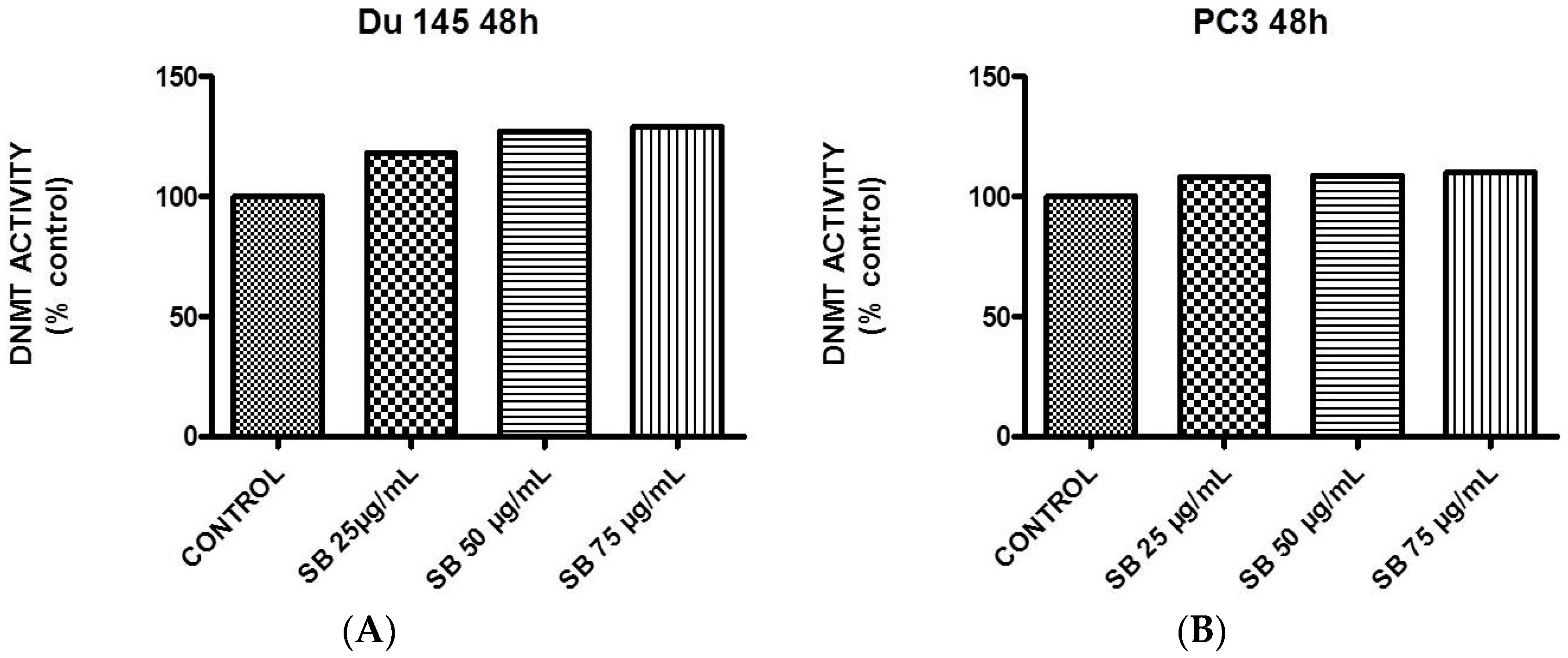
2.5. Silibinin Causes a Modest Concentration-Dependent Increase in DNA Methyltransferase Activity
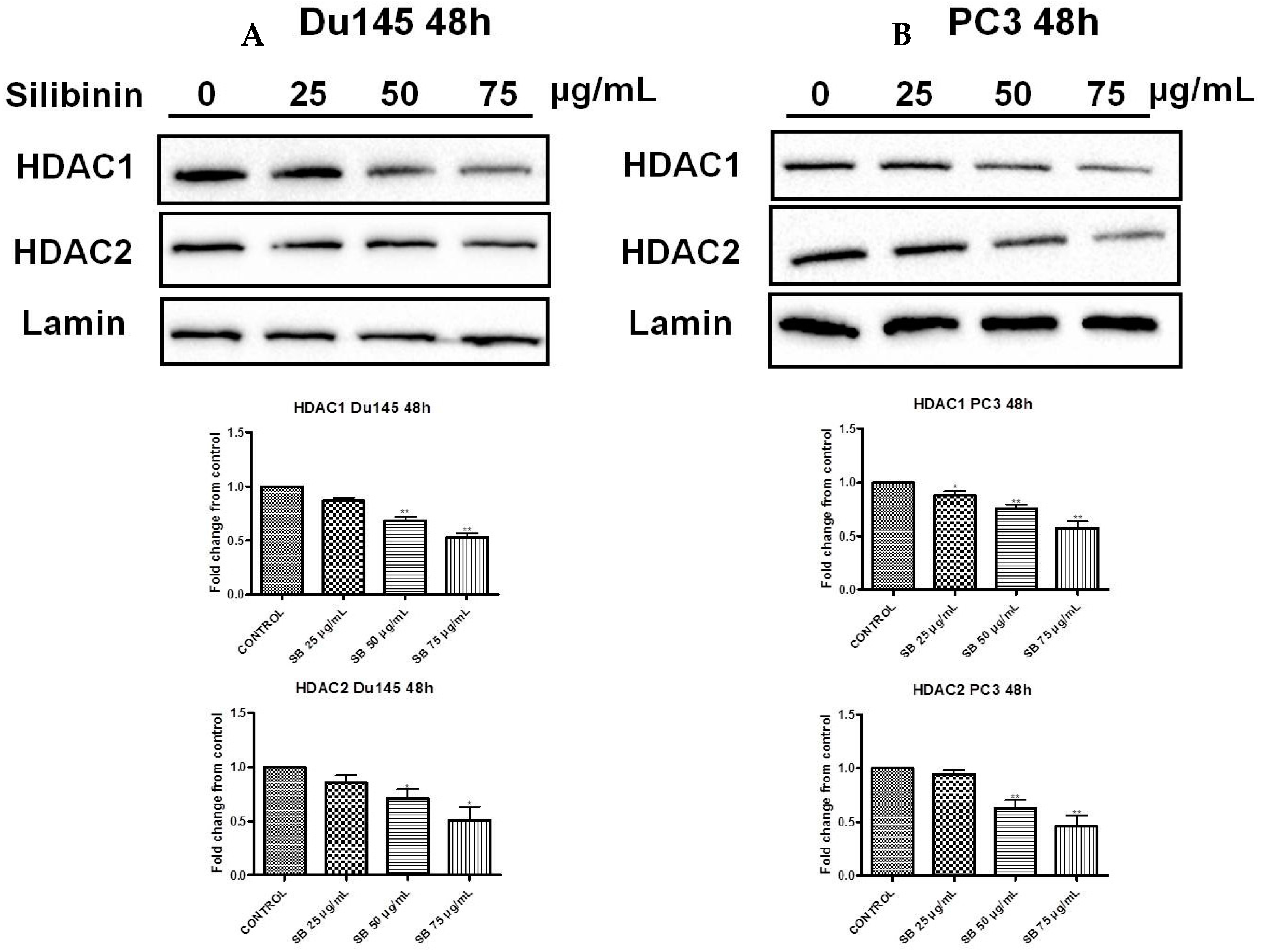
2.6. Silibinin Causes Concentration-Dependent Decrease of HDAC1-2 Expression Levels
3. Discussion
4. Materials and Methods
4.1. Cell lines and Treatments
4.2. Reagents and Antibodies
4.3. Protein Extraction, Cell Lysates Preparation and Western Immunoblotting
4.4. RT-PCR Methodology
4.5. Confocal Immunofluorescence Protocol
4.6. DNA Methyltransferase Activity Assay
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Chiam, K.; Ricciardelli, C.; Bianco-Miotto, T. Epigenetic biomarkers in prostate cancer: Current and future uses. Cancer Lett. 2014, 342, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.P.; Dickinson, J.L.; Holloway, A.F. Epigenetic regulation of prostate cancer. Clin. Epigenet. 2011, 2, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.A.; Yu, J. EZH2, an epigenetic driver in prostate cancer. Protein Cell 2013, 4, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ngollo, N.; Dagdemir, A.; Karsli-Ceppioglu, S.; Judes, G.; Pajon, A.; Penault-Llorca, F.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D.J. Epigenetic modifications in prostate cancer. Epigenomics 2014, 6, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Buckles, E.; Estrada, J.; Koochekpour, S. Aberrant DNA methylation and prostate cancer. Curr. Genom. 2011, 12, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.Y.; Yu, S.N.; Park, S.K.; Choi, H.D.; Ji, J.H.; Ahn, S.C. Autophagy inhibition enhances silibinin-induced apoptosis by regulating reactive oxygen species production in human prostate cancer PC-3 cells. Biochem. Biophys. Res. Commun. 2015, 468, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kroll, D.J.; Shaw, H.S.; Oberlies, N.H. Milk thistle nomenclature: Why it matters in cancer research and pharmacokinetic studies. Integr. Cancer Ther. 2007, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.; Deep, G.; Agarwal, R. Molecular mechanisms of silibinin-mediated cancer chemoprevention with major emphasis on prostate cancer. AAPS J. 2013, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.; Deep, G.; Agarwal, C.; Agarwal, R. The strategies to control prostate cancer by chemoprevention approaches. Mutat. Res. 2014, 760, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deb, G.; Thakur, V.S.; Gupta, S. Multifaceted role of EZH2 in breast and prostate cancer. Epigenetics 2013, 8, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Karanikolas, B.D.; Figueiredo, M.L.; Wu, L. Polycomb group protein enhancer of zeste 2 is an oncogene that promotes the neoplastic transformation of a benign prostatic epithelial cell line. Mol. Cancer Res. 2009, 7, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.B. A gene complex controlling segmentation in Drosophila. Nature 1978, 276, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Viré, E.; Brenner, C.; Deplus, R.; Blachon, L.; Fraga, M.; Didelot, C.; Morey, L.; van Eynde, A.; Bernard, D.; Vanderwinden, J.M.; et al. The polycomb group protein EZH2 directly controls the DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, N.D.; Yee, D.; Chen, A.; Kalantry, S.; Chamberlain, S.J.; Otte, A.P.; Magnuson, T. The murine polycomb protein EED is required for global histone H3-lysine 27 methylation. Curr. Biol. 2005, 15, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vlag, J.; Otte, A.P. Transcriptional repression mediated by the human polycomb-group EED involves histone deacetylation. Nat. Genet. 1999, 23, 474–478. [Google Scholar] [PubMed]
- Chase, A.; Cross, N.C. Aberrations of EZH2 in cancer. Clin. Cancer Res. 2011, 17, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, I.; Bagella, L. Targeting Enhancer of Zeste Homolog 2 as a promising strategy for cancer treatment. World J. Clin. Oncol. 2016, 7, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Varambally, S.; Dhanasekaran, S.M.; Zhon, M.; Kumar-Sihna, C.; Sanda, M.G.; Ghosh, P.; Pienta, K.J.; Sewalt, R.G.; Otte, A.P.; Rubin, M.A.; et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, I.M.; Halvorsen, O.J.; Collet, K.; Stefansson, I.M.; Collet, K.; Straume, O.; Haukaass, S.A.; Salvesen, H.B.; Otte, A.P.; Akslen, L.A. EZH2 expression is associated with high proliferation rates and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrial, prostate, and breast. J. Clin. Oncol. 2006, 24, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Hurt, M.; Mathews, C.A.; Cabarcas, S.M.; Sun, L.; Marquez, V.E.; Danesi, R.; Farrar, W.L. Pharmacologic disruption of polycomb repressive complex 2 inhibits tumorigenicity and tumor progression in prostate cancer. Mol. Cancer 2011, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Jain, O.; di Croce, L. Mutations and deletions of PRC2 in prostate cancer. Bioessays 2016, 38, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Gustafson, D.L.; Su, L.J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.L.; Zhou, B.P.; Xia, W.; Wu, Y.; Yang, C.C.; Chen, C.T.; Ping, B.; Otte, A.P.; Hung, M.C. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 2005, 310, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Graça, I.; Pereira-Silva, E.; Henrique, R.; Packham, G.; Crabb, S.J.; Jerónimo, C. Epigenetic modulators as therapeutic targets in prostate cancer. Clin. Epigenet. 2016, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Roske, A.; Gekeler, V.; Beckers, T.; Stephan, C.; Jung, K.; Fritzsche, F.R.; Niesporek, S.; Denkert, C.; Dietel, M.; et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer 2008, 98, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Deb, G.; Singh, A.K.; Gupta, S. EZH2: Not EZHY (easy) to deal. Mol. Cancer Res. 2014, 12, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.K.; Mao, X.; Lu, Y.J. The complexity of prostate cancer: Genomic alterations and heterogeneity. Nat. Rev. Urol. 2012, 9, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, C.; Henrique, R. Epigenetic biomarkers in urological tumors: A systematic review. Cancer Lett. 2014, 342, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Anestopoulos, I.; Voulgaridou, G.P.; Georgakilas, A.G.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Epigenetic therapy as a novel approach in hepatocellular carcinoma. Pharmacol. Ther. 2015, 145, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lange, C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. 2008, 647, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Koppens, M.; van Lohuizen, M. Context-dependent actions of Polycomb repressors in cancer. Oncogene 2016, 35, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenet. 2015, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, N.; Johnson, D.W.; Nicol, D.L. Silibinin—A promising new treatment for cancer. Anticancer Agents Med. Chem. 2010, 10, 186–195. [Google Scholar]
- Flaig, T.W.; Glodé, M.; Gustafson, D.; van Bokhoven, A.; Tao, Y.; Wilson, S.; Su, L.J.; Li, Y.; Harrison, G.; Agarwal, R.; et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate 2010, 70, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Agarwal, C.; Agarwal, R. The cancer preventive flavonoid silibinin causes hypophosphorylation of Rb/p107 and Rb2/p130 via modulation of cell cycle regulators in human prostate carcinoma DU145 cells. Cell Cycle 2002, 1, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Agarwal, C.; Agarwal, R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: Role in prostate cancer prevention. Mol. Cancer Ther. 2002, 1, 525–532. [Google Scholar] [PubMed]
- Zi, X.; Zhang, J.; Agarwal, R.; Pollak, M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000, 60, 5617–5620. [Google Scholar] [PubMed]
- Bracken, A.P.; Pasini, D.; Capra, M.; Prosperini, E.; Colli, E.; Helin, K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003, 22, 5323–5335. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, X.; Zhuang, L.; Jiang, X.; Chen, W.; Lee, P.L.; Karuturi, R.K.; Tan, P.B.; Liu, E.T.; Yu, Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007, 21, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, C.W.; Yu, C.C.; Wang, B.Y.; Huang, Y.H.; Hsieh, Y.C.; Kuo, Y.L.; Chang, W.W. Resveratrol suppresses myofibroblast activity of human buccal mucosal fibroblasts through the epigenetic inhibition of ZEB1 expression. Oncotarget 2016, 7, 12137–12149. [Google Scholar] [PubMed]
- Selth, L.A.; Das, R.; Townley, S.L.; Coutinho, I.; Hanson, A.R.; Centenera, M.M.; Stylianou, N.; Sweeney, K.; Soekmadji, C.; Jovanovic, L.; et al. A ZEB1-miR-375-YAP1 pathway regulates epithelial plasticity in prostate cancer. Oncogene 2016. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Lee, J.H.; Han, H.H.; Kim, B.G.; Kang, S.; Choi, Y.D.; Cho, N.H. MicroRNA alteration and putative target genes in high-grade prostatic intraepithelial neoplasia and prostate cancer: STAT3 and ZEB1 are upregulated during prostate carcinogenesis. Prostate 2016, 76, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zeng, J.; Li, L.; Fan, J.; Zhang, D.; Xue, Y.; Zhu, G.; Yang, L.; Wang, X.; He, D. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol. Rep. 2010, 23, 1545–1552. [Google Scholar] [PubMed]
- Bosch-Barrera, J.; Menendez, J.A. Silibinin and STAT3: A natural way of targeting transcription factors for cancer therapy. Cancer Treat. Rev. 2015, 41, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xia, W.; Zhang, Z.; Liu, J.; Wang, H.; Adsay, N.V.; Albarracin, C.; Yu, D.; Abbruzzese, J.L.; Mills, G.B.; et al. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol. Carcinog. 2008, 47, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Rogenhofer, S.; Kahl, P.; Mertens, C.; Hauser, S.; Hartmann, W.; Büttner, R.; Müller, S.C.; von Ruecker, A.; Ellinger, J. Global histone H3 lysine 27 (H3K27) methylation levels and their prognostic relevance in renal cell carcinoma. BJU Int. 2012, 109, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, N.; Matsumoto, K.; Nanashima, A.; Nagayasu, T.; Hayashi, T.; Ying, M.; Endo, D.; Wu, Z.; Koji, T. High expression of trimethylated histone H3 at lysine 27 predicts better prognosis in non-small cell lung cancer. Int. J. Oncol. 2013, 43, 1467–1480. [Google Scholar] [PubMed]
- Tzao, C.; Tung, H.J.; Jin, J.S.; Sun, G.H.; Hsu, H.S.; Chen, B.H.; Yu, C.P.; Lee, S.C. Prognostic significance of global histone modifications in resected squamous cell carcinoma of the esophagus. Mod. Pathol. 2009, 22, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Y.; Tong, Z.T.; Zhu, W.; Wen, Z.Z.; Rao, H.L.; Kong, L.L.; Guan, X.Y.; Kung, H.F.; Zeng, Y.X.; Xie, D. H3K27me3 protein is a promising predictive biomarker of patients’ survival and chemoradioresistance in human nasopharyngeal carcinoma. Mol. Med. 2011, 17, 1137–1145. [Google Scholar] [PubMed]
- Cai, M.Y.; Hou, J.H.; Rao, H.L.; Luo, R.Z.; Li, M.; Pei, X.Q.; Lin, M.C.; Guan, X.Y.; Kung, H.F.; Zeng, Y.X.; et al. High expression of H3K27me3 in human hepatocellular carcinomas correlates closely with vascular invasion and predicts worse prognosis in patients. Mol. Med. 2011, 17, 12–20. [Google Scholar] [PubMed]
- Cao, R.; Zhang, Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 2004, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Kahl, P.; von der Gathen, J.; Heukamp, L.C.; Gütgemann, I.; Walter, B.; Hofstädter, F.; Bastian, P.J.; von Ruecker, A.; Müller, S.C.; et al. Global histone H3K27 methylation levels are different in localized and metastatic prostate cancer. Cancer Investig. 2012, 30, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Deligezer, U.; Yaman, F.; Darendeliler, E.; Dizdar, Y.; Holdenrieder, S.; Kovancilar, M.; Dalay, N. Post-treatment circulating plasma BMP6 mRNA and H3K27 methylation levels discriminate metastatic prostate cancer from localized disease. Clin. Chim. Acta 2010, 411, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Ngollo, M.; Lebert, A.; Dagdemir, A.; Judes, G.; Karsli-Ceppioglu, S.; Daures, M.; Kemeny, J.L.; Penault-Llorca, F.; Boiteux, J.P.; Bignon, Y.J.; et al. The association between histone 3 lysine 27 trimethylation (H3K27me3) and prostate cancer: Relationship with clinicopathological parameters. BMC Cancer 2014, 14, 994. [Google Scholar] [CrossRef] [PubMed]
- Ngollo, M.; Dagdemir, A.; Judes, G.; Kemeny, J.L.; Penault-Llorca, F.; Boiteux, J.P.; Lebert, A.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D. Epigenetics of prostate cancer: Distribution of histone H3K27me3 biomarkers in peri-tumoral tissue. OMICS 2014, 18, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Pellakuru, L.G.; Iwata, T.; Gurel, B.; Schultz, D.; Hicks, J.; Bethel, C.; Yegnasubramanian, S.; de Marzo, A.M. Global levels of H3K27me3 track with differentiation in vivo and are deregulated by MYC in prostate cancer. Am. J. Pathol. 2012, 181, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.M.; Iwata, T.; Zheng, Q.; Bethel, C.; Yegnasubramanian, S.; de Marzo, A.M. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget 2011, 2, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Wang, J.; Yumoto, K.; Jung, Y.; Cackowski, F.C.; Decker, A.M.; Li, Y.; Franceschi, R.T.; Pienta, K.J.; Taichman, R.S. DNMT1 Regulates Epithelial-Mesenchymal Transition and Cancer Stem Cells, Which Promotes Prostate Cancer Metastasis. Neoplasia 2016, 18, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Kauntz, H.; Bousserouel, S.; Gossé, F.; Raul, F. Epigenetic effects of the natural flavonolignan silibinin on colon adenocarcinoma cells and their derived metastatic cells. Oncol. Lett. 2013, 5, 1273–1277. [Google Scholar] [PubMed]
- Lah, J.J.; Cui, W.; Hu, K.Q. Effects and mechanisms of silibinin on human hepatoma cell lines. World J. Gastroenterol. 2007, 13, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Gu, F.; Hu, K.Q. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J. Gastroenterol. 2009, 15, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Raina, K.; Jain, A.K.; Agarwal, C.; Chan, D.; Agarwal, R. Epigenetic modifications and p21-cyclin B1 nexus in anticancer effect of histone deacetylase inhibitors in combination with silibinin on non-small cell lung cancer cells. Epigenetics 2012, 7, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Raina, K.; Agarwal, C.; Chan, D. Silibinin synergizes with histone deacetylase and DNA methyltransferase inhibitors in upregulating E-cadherin expression together with inhibition of migration and invasion of human non-small cell lung cancer cell. J. Pharmacol. Exp. Ther. 2013, 345, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Marjanovic, N.D.; Lee, T.; Bell, G.; Kleer, C.G.; Reinhardt, F.; D’Alessio, A.C.; Young, R.A.; Weinberg, R.A. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 2013, 154, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Silibinin is available from Sigma-Aldrich (Cat. No. S0417).
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anestopoulos, I.; Sfakianos, A.P.; Franco, R.; Chlichlia, K.; Panayiotidis, M.I.; Kroll, D.J.; Pappa, A. A Novel Role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma. Molecules 2017, 22, 62. https://doi.org/10.3390/molecules22010062
Anestopoulos I, Sfakianos AP, Franco R, Chlichlia K, Panayiotidis MI, Kroll DJ, Pappa A. A Novel Role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma. Molecules. 2017; 22(1):62. https://doi.org/10.3390/molecules22010062
Chicago/Turabian StyleAnestopoulos, Ioannis, Aristeidis P. Sfakianos, Rodrigo Franco, Katerina Chlichlia, Mihalis I. Panayiotidis, David J. Kroll, and Aglaia Pappa. 2017. "A Novel Role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma" Molecules 22, no. 1: 62. https://doi.org/10.3390/molecules22010062





