Synthesis of New Nitrofluoroquinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant H. pylori
Abstract
:1. Introduction
2. Results and Discussion
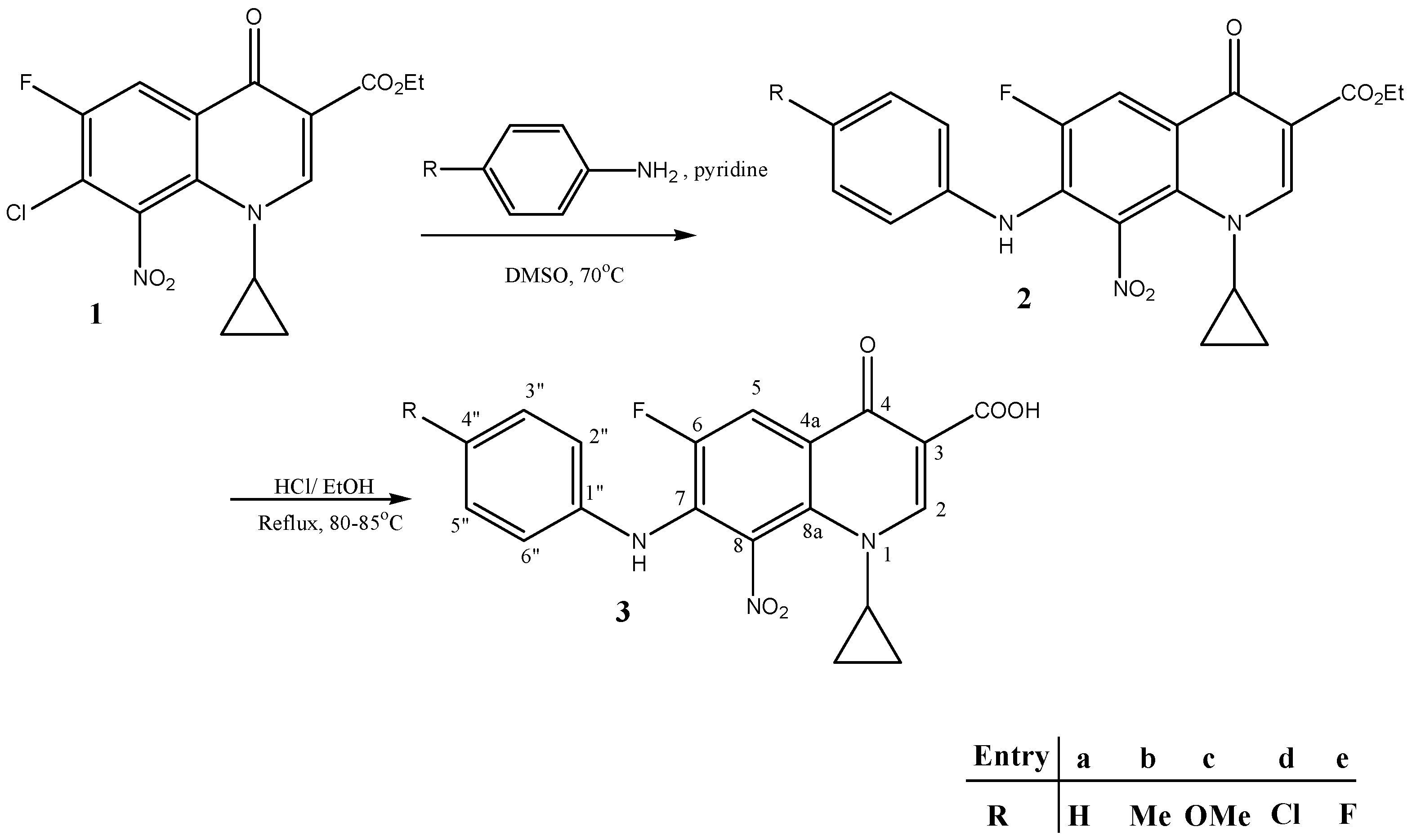
2.1. Synthesis of New Compounds
2.2. Antimicrobial Activity against Metronidazole-Resistant H. pylori
2.3. Inhibitory Effects of the Synthesized Compounds against H. pylori Urease Enzyme
2.4. Structural Activity Relationship Studies
3. Materials and Methods
3.1. Materials and Instruments
3.1.1. Synthesis of Novel Compounds 3b and 3c
Synthesis of Compound 1
Synthesis of New Compounds (Scheme 1)
3.2. Microbiological Methods and Anti-Microbial Assays
3.2.1. Bacterial Strains and Growth Conditions
3.2.2. Antimicrobial Susceptibility Testing and Minimal Inhibitory Concentration Determination
3.2.3. Determination of in Vitro Interaction
- FIC = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone).
- The FIC indices were interpreted as follows: ≤0.5, synergy; 0.5–1, additive; 1–4.0, indifference; >4.0, antagonism.
3.3. Urease Inhibition Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hayama, M.; Kawakami, Y.; Kaneko, Y.; Sano, K.; Ota, H. Helicobacter pylori infection increases cell kinetics in human gastric epithelial cells without adhering to proliferating cells. J. Cell Mol. Med. 2005, 9, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Kalali, B.; Mejías-Luque, R.; Javaheri, A.; Gerhard, M. H. pylori virulence factors: Influence on immune system and pathology. Mediat. Inflamm. 2014, 2014, 6642–6651. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Ballal, M.; Lingadakai, R.; Mukhopadhyay, A. Determination of Helicobacter pylori virulence genes in clinical isolates of symptomatic patients from South Coastal Region of Karnataka—A preliminary work. Austin J. Gastroenterol. 2015, 2, 1031. [Google Scholar]
- Marshall, B.J.; Armstrong, J.A.; McGechie, D.B.; Glancy, R.J. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med. J. Aust. 1985, 142, 436–439. [Google Scholar] [PubMed]
- Mendes, L.T.; Attygalle, A.D.; Wotherspoon, A.C. Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: A re-evaluation. Gut. 2014, 63, 1526–1527. [Google Scholar] [CrossRef] [PubMed]
- Asaka, M.; Sugiyama, T.; Kato, M.; Satoh, K.; Kuwayama, H.; Fukuda, Y.; Fujioka, T.; Takemoto, T.; Kimura, K.; Shimoyama, T.; et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 2001, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Molina-Infante, J.; Gisbert, J.P.; O’Morain, C. Treatment of Helicobacter pylori infection. Helicobacter 2013, 18, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Choudhary, A.; Bechtold, M.L. Effect of Helicobacter pylori treatment on gastroesophageal reflux disease (GERD): Meta-analysis of randomized controlled trials. Scand. J. Gastroenterol. 2012, 47, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Giangaspero, A.; Losurdo, G.; Giorgio, F.; Amoruso, A.; de Francesco, V. Quadruple rescue therapy after first and second line failure for Helicobacter pylori treatment: Comparison between two tetracycline-based regimens. J. Gastrointestin. Liver Dis. 2014, 23, 367–370. [Google Scholar] [PubMed]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.; Albrecht, P. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Andrews, J.M.; Edwards, L.J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob. Agents Chemother. 1983, 23, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Felmingham, D.; O’Hare, M.D.; Robbins, M.J.; Wall, R.A.; Williams, A.H.; Cremer, A.W.; Ridgeway, G.L.; Gruneberg, R.N. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs Exp. Clin. Res. 1985, 11, 317–329. [Google Scholar] [PubMed]
- Maurer, F.; Grohe, K. 2,4-Dichloro-5-fluorobenzoic acid. Chem. Abstr. 1986, 105, 97158e. [Google Scholar]
- Petersen, U.; Bartel, S.; Bremm, K.D.; Himmler, T.; Krebs, A.; Schenke, T. The synthesis and biological properties of 6-fluoroquinolonecarboxylic acids. Bull. Soc. Chim. Belg. 1996, 105, 683–699. [Google Scholar]
- Vilaichone, R.K.; Gumnarai, P.; Ratanachu-ek, T.; Mahachai, V. Nationwide survey of Helicobacter pylori antibiotic resistance in Thailand. Diagn. Microbiol. Infect. Dis. 2013, 77, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Sheng, W.H.; Liou, J.M.; Wang, H.P.; Wu, M.S.; Lin, J.T.; Chang, S.C. Comparative in vitro antimicrobial susceptibility and synergistic activity of antimicrobial combinations against Helicobacter pylori isolates in Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiari, Y.M.; Qandil, A.M.; Al-Zoubi, R.M.; Alzweiri, M.H.; Darwish, R.M.; Shattat, G.F.; Al-Qirim, T.M. Synthesis and antibacterial activity of novel 7-haloanilino-8-nitrofluoroquinolone derivatives. Med. Chem. Res. 2012, 21, 1734–1740. [Google Scholar] [CrossRef]
- Al-Hiari, Y.M.; Al-Mazari, I.S.; Shakya, A.K.; Darwish, R.M.; Abu-Dahab, R. Synthesis and antibacterial properties of new 8-nitro fluoroquinolone derivatives. Molecules 2007, 12, 1240–1258. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F.; El-Omar, E.M. Management of Helicobacter pylori infection—The Maastricht IV/Florence consensus report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Qatouseh, L.F.; Boutennone, H.; Boussouf, L.; Madani, K.; Shihab, P.; Al-Qaoud, K. In Vitro anti-Helicobacter pylori and urease inhibitory effects of polyphenolic extracts of local herbs from Algeria. IAJAA 2014, 3, 1–4. [Google Scholar]
- Yakoob, J.; Abbas, Z.; Khan, R.; Naz, S.; Ahmad, Z.; Islam, M.; Awan, S.; Jafri, F.; Jafri, W. Prevalence of non Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Klancnik, A.; Piskernik, S.; Jersek, B.; Mozina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Inoue, H.; Ishii, C.; Okazaki, Y.; Domon, H.; Utsui, Y. Effect of plaunotol in combination with clarithromycin or amoxicillin on Helicobacter pylori in vitro and in vivo. J. Antimicrob. Chemother. 2002, 50, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Mizuta, T.; Tonokatu, Y.; Fukuda, Y.; Okamura, H.; Hayashi, T.; Tamura, T. Monoclonal Antibodies against the Native Urease of Helicobacter pylori: Synergistic Inhibition of Urease Activity by Monoclonal Antibody Combinations. Infect. Immun. 1992, 60, 4826–4831. [Google Scholar] [PubMed]
- SampleAvailability: Samples of the compounds are not available from the authors.
| Tested Compound | Zones of Inhibition (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Isolates | Control Strain | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 3a | 10 | 20 | 15 | 10 | 20 | 11 | 11 | 17 | 9 | 15 | 13 | 15 | 18 |
| 3b | 12 | 12 | 0 | 15 | 11 | 16 | 13 | 15 | 11 | 12 | 14 | 13 | 12 |
| 3c | 20 | 24 | 20 | 16 | 24 | 20 | 18 | 25 | 15 | 20 | 23 | 25 | 25 |
| 3d | 10 | 10 | 8 | 12 | 13 | 11 | 14 | 15 | 9 | 14 | 13 | 12 | 12 |
| 3e | 10 | 12 | 0 | 15 | 15 | 14 | 12 | 16 | 9 | 15 | 15 | 14 | 13 |
| CIP | 20 | 40 | 0 | 40 | 50 | 45 | 50 | 0 | 0 | 45 | 50 | 45 | 45 |
| MTZ | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Compounds | Minimum Inhibitory Concentration (µg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain Number | Control Strain | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 3a | 32 | 16 | 16 | 16 | 8 | 32 | 16 | 8 | 16 | 32 | 16 | 16 | 16 |
| 3b | 32 | 32 | 16 | 32 | 32 | 64 | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| 3c | 4 | 4 | 8 | 4 | 2 | 4 | 4 | 8 | 4 | 4 | 8 | 4 | 8 |
| 3d | 32 | 16 | 16 | 16 | 32 | 32 | 16 | 16 | 32 | 32 | 16 | 32 | 16 |
| 3e | 16 | 32 | 32 | 16 | 32 | 32 | 16 | 32 | 32 | 32 | 16 | 32 | 32 |
| CIP | 0.3 | 0.6 | 0.3 | 0.6 | 0.3 | 0.04 | 0.6 | 0.04 | 0.08 | 0.04 | 0.6 | 0.6 | 0.6 |
| MTZ | 64 | 64 | 64 | 32 | 128 | 64 | 128 | 32 | 64 | 64 | 128 | 64 | 128 |
| Compounds | FIC Values (Index) (MIC Combination) | FIC Mean | ||
|---|---|---|---|---|
| Strain Number | ||||
| 11 | 12 | Control Strain | ||
| 3a-MTZ | 0.328 (*) | 0.328 (*) | 0.328 (*) | 0.328 (*) |
| (2.5) | (2.5) | (2.5) | ||
| 3b-MTZ | 1.312 (=) | 1.312 (=) | 1.312 (=) | 1.312 (=) |
| (10) | (10) | (10) | ||
| 3c-MTZ | 0.656 (+) | 0.656 (+) | 0.656 (+) | 0.656 (+) |
| (5) | (5) | (5) | ||
| 3d-MTZ | 0.328 (*) | 0.328 (*) | 0.328 (*) | 0.328 (*) |
| (2.5) | (2.5) | (2.5) | ||
| 3e-MTZ | 1.312 (=) | 0.656 (+) | 0.656 (+) | 0.874 (+) |
| (10) | (5) | (5) | ||
| Compound | IC50 (µM) |
|---|---|
| 3a | NS |
| 3b | 629 |
| 3c | 151 |
| 3d | NS |
| 3e | NS |
| AHA | 27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Qatouseh, L.; Abu-Sini, M.; Mayyas, A.; Al-Hiari, Y.; Darwish, R.; Aburjai, T. Synthesis of New Nitrofluoroquinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant H. pylori. Molecules 2017, 22, 71. https://doi.org/10.3390/molecules22010071
Abu-Qatouseh L, Abu-Sini M, Mayyas A, Al-Hiari Y, Darwish R, Aburjai T. Synthesis of New Nitrofluoroquinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant H. pylori. Molecules. 2017; 22(1):71. https://doi.org/10.3390/molecules22010071
Chicago/Turabian StyleAbu-Qatouseh, Luay, Mohammad Abu-Sini, Amal Mayyas, Yusuf Al-Hiari, Rula Darwish, and Talal Aburjai. 2017. "Synthesis of New Nitrofluoroquinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant H. pylori" Molecules 22, no. 1: 71. https://doi.org/10.3390/molecules22010071





