Effect of Phenolic Compounds from Elderflowers on Glucose- and Fatty Acid Uptake in Human Myotubes and HepG2-Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Uptake of Glucose in Human Skeletal Muscle Cells and HepG2-Cells
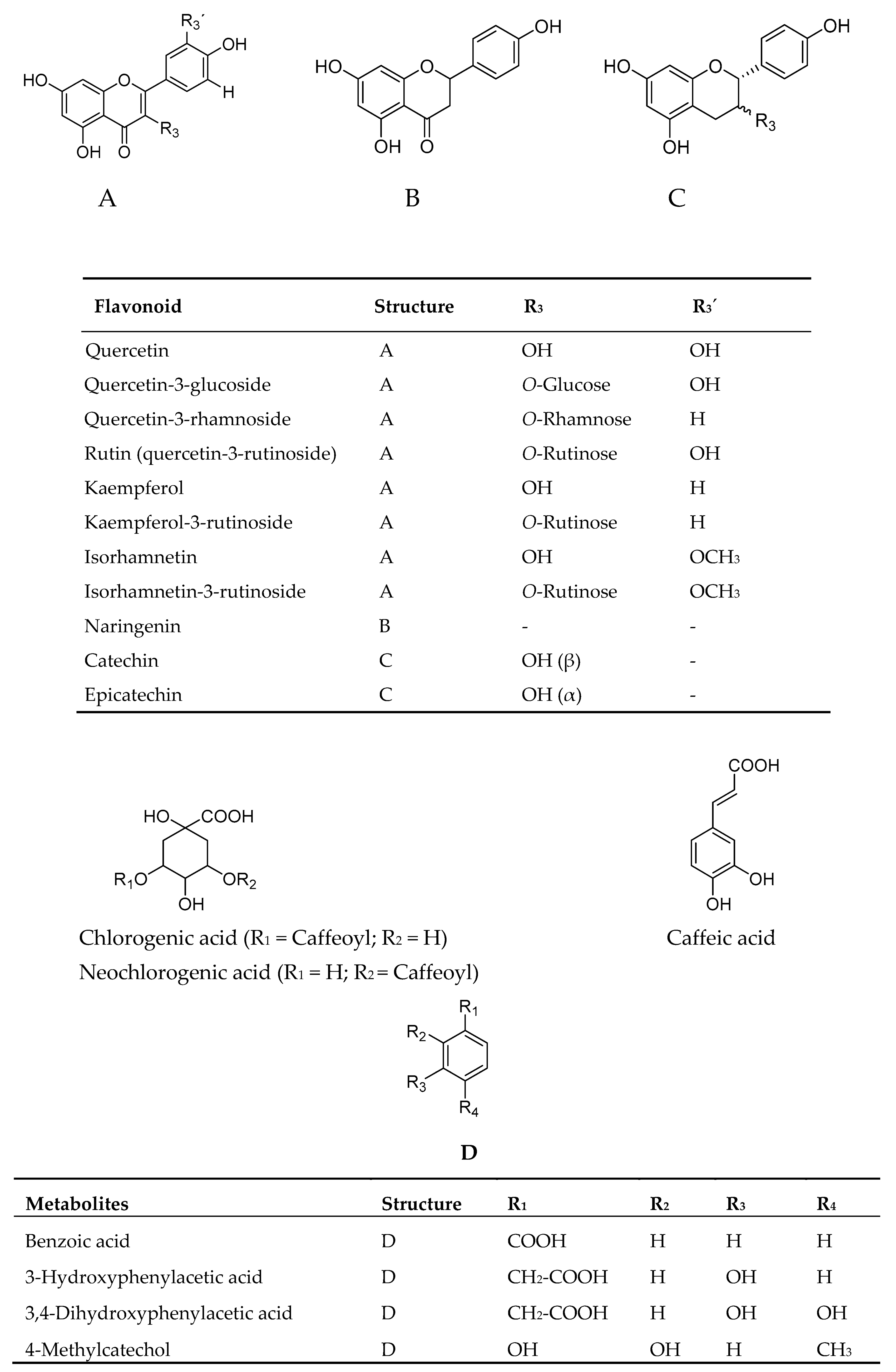
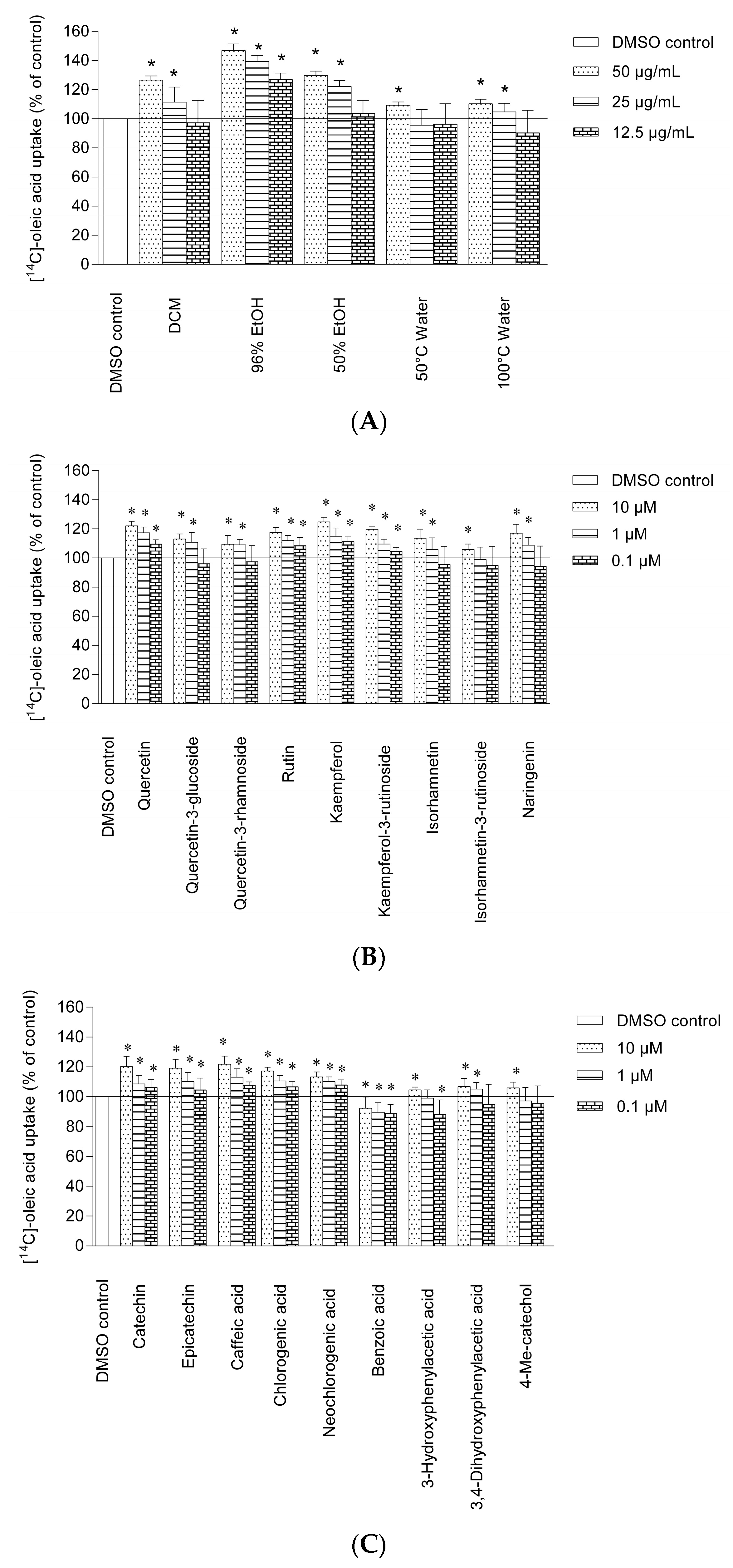
2.2. Uptake of Oleic Acid in Human Skeletal Muscle Cells and HepG2-cells
2.3. α-Amylase and α-Glucosidase Inhibitory Activity
2.4. Free Radicals and Antioxidant Activities
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Extraction
3.4. NMR
3.5. Culturing of Human Myotubes
3.6. Culturing of HepG2-Cells
3.7. Glucose and Oleic Acid Uptake
3.8. α-Glucosidase Inhibitory Activity
3.9. α-Amylase Inhibitory Activity
3.10. DPPH Radical Scavenging
3.11. Inhibition of 15-Lipoxygenase
3.12. Inhibition of Xanthine Oxidase
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skyler, J.S. Diabetes mellitus: Pathogenesis and treatment strategies. J. Med. Chem. 2004, 47, 4113–4117. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine: Expanded Commission E Monographs; American Botanical Council: Austin, TX, USA, 2000. [Google Scholar]
- Weiss, R.; Fintelmann, V. Herbal Medicine; Thieme: Stuttgart, Germany, 2000. [Google Scholar]
- Ho, G.T.T.; Zou, Y.-F.; Aslaksen, T.H.; Wangensteen, H.; Barsett, H. Structural characterization of bioactive pectic polysaccharides from elderflowers (Sambuci flos). Carbohydr. Polym. 2016, 135, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Zou, Y.-F.; Wangensteen, H.; Barsett, H. RG-I regions from elderflower pectins substituted on GalA are strong immunomodulators. Int. J. Biol. Macromol. 2016, 92, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Beaux, D.; Fleurentin, J.; Mortier, F. Effect of extracts of Orthosiphon stamineus benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi L. spreng. in rats. Phytother. Res. 1999, 13, 222–225. [Google Scholar] [CrossRef]
- Gray, A.M.; Abdel-Wahab, Y.H.; Flatt, P.R. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J. Nutr. 2000, 130, 15–20. [Google Scholar] [PubMed]
- Groop, L.C.; Bonadonna, R.C.; DelPrato, S.; Ratheiser, K.; Zyck, K.; Ferrannini, E.; DeFronzo, R.A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Investig. 1989, 84, 205. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Christensen, K.B.; Olsen, L.C.; Christensen, L.P.; Grevsen, K.; Færgeman, N.J.; Kristiansen, K.; Young, J.F.; Oksbjerg, N. Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 11033–11040. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) γ. Phytother. Res. 2010, 24 (Suppl. 2), S129–S132. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Minet, A.; Svenstrup, H.; Grevsen, K.; Zhang, H.; Schrader, E.; Rimbach, G.; Wein, S.; Wolffram, S.; Kristiansen, K. Identification of plant extracts with potential antidiabetic properties: Effect on human peroxisome proliferator-activated receptor (PPAR), adipocyte differentiation and insulin-stimulated glucose uptake. Phytother. Res. 2009, 23, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. BBA Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dürüst, N.; Özden, S.; Umur, E.; Dürüst, Y.; Kucukislamoglu, M. The isolation of carboxylic acids from the flowers of Delphinium formosum. Turk. J. Chem. 2001, 25, 93–97. [Google Scholar]
- Napolitano, J.G.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Complete 1H NMR spectral analysis of ten chemical markers of Ginkgo biloba. Magn. Reson. Chem. 2012, 50, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional elderflower beverages: A rich source of phenolic compounds with high antioxidant activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-K.; Gao, J.; Zhu, D.-N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Zhang, W.Y.; Jin, W.; Lee, I.S.; Min, B.-S.; Jung, H.-J.; Na, M.; Lee, S.; Bae, K. Flavonoids and isoflavonoids from Sophorae Flos improve glucose uptake in vitro. Planta Med. 2010, 76, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; McInerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G. Diabetes and the Mediterranean diet: A beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM Int. J. Med. 2000, 93, 85–91. [Google Scholar] [CrossRef]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.S.; Barsett, H. Bioactive Pectic Polysaccharides; Springer: Berlin, Germany, 2005. [Google Scholar]
- Kim, M. High-methoxyl pectin has greater enhancing effect on glucose uptake in intestinal perfused rats. Nutrition 2005, 21, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, T.; Cao, H. Flavonoid glycosylation and biological benefits. Biotechnol. Adv. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Manaharan, T.; Appleton, D.; Cheng, H.M.; Palanisamy, U.D. Flavonoids isolated from Syzygium aqueum leaf extract as potential antihyperglycaemic agents. Food Chem. 2012, 132, 1802–1807. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. α-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadi, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitaminol. 2007, 53, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L.(antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Sadeghian, H.; Jabbari, A. 15-Lipoxygenase inhibitors: A patent review. Expert Opin. Ther. Pat. 2016, 26, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.N.A.; Tako, M.; Hanashiro, I.; Tamaki, H. Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem. 2008, 109, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kase, E.T.; Nikolić, N.; Hessvik, N.P.; Fjeldheim, Å.-K.; Jensen, J.; Thoresen, G.H.; Rustan, A.C. Dietary supplementation with 22-S-hydroxycholesterol to rats reduces body weight gain and the accumulation of liver triacylglycerol. Lipids 2012, 47, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.-H.; Hwang, H.-J.; Sung, H.-C. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J. Agric. Food Chem. 2001, 49, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Kayano, S.-I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus d omestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Kase, E.T.; Wensaas, A.J.; Aas, V.; Højlund, K.; Levin, K.; Thoresen, G.H.; Beck-Nielsen, H.; Rustan, A.C.; Gaster, M. Skeletal muscle lipid accumulation in type 2 diabetes may involve the liver X receptor pathway. Diabetes 2005, 54, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Wensaas, A.; Rustan, A.; Lövstedt, K.; Kull, B.; Wikström, S.; Drevon, C.; Hallen, S. Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J. Lipid Res. 2007, 48, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Gella, F.-J.; Gubern, G.; Vidal, R.; Canalias, F. Determination of total and pancreatic α-amylase in human serum with 2-chloro-4-nitrophenyl-α-d-maltotrioside as substrate. Clin. Chim. Acta 1997, 259, 147–160. [Google Scholar] [CrossRef]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.

| Elderflower Extract | DPPH (µg/mL) | 15-LO (µg/mL) | XO (µg/mL) | α-Glucosidase (µg/mL) | α-Amylase (µg/mL) |
|---|---|---|---|---|---|
| DCM | >167 | 125.9 ± 3.9 | >167 | 105 ± 5.6 | 103 ± 5.9 |
| 96% EtOH | 9.2 ± 0.9 | 17.9 ± 3.6 | 59.3 ± 6.3 | 4.8 ± 0.5 | 2.8 ± 1.1 |
| 50% EtOH | 20.2 ± 3.9 | 24.4 ± 3.1 | 79.5 ± 4.1 | 8.9 ± 1.1 | 3.1 ± 1.3 |
| 50 °C Water | 68.9 ± 2.3 | 126.6 ± 3.9 | 156.5 ± 5.3 | 78.9 ± 5.8 | 71.8 ± 4.1 |
| 100 °C Water | 32.0 ± 2.9 | 75.9 ± 6.5 | 135.6 ± 7.8 | 65.3 ± 4.6 | 66.2 ± 5.6 |
| Quercetin (control) | 2.8 ± 0.3 | 29.3 ± 1.9 | 0.7 ± 0.2 | nt | nt |
| Acarbose (control) | nt | nt | nt | 84.7 ± 3.8 | 73.3 ± 4.3 |
| Test Compound | DPPH 1 (µM) | 15-LO 1 (µM) | XO 1 (µM) | α-Glucosidase 2 (µM) | α-Amylase 3 (µM) |
|---|---|---|---|---|---|
| Phenolic Compounds | |||||
| Quercetin 1 | 9.3 ± 1.5 | 95.9 ± 1.3 | 2.3 ± 0.3 | 2.6 ± 0.9 | 2.1 ± 0.5 |
| Quercetin-3-glucoside | 17.6 ± 3.2 | 102.3 ± 5.3 | 105.9 ± 5.3 | 4.1 ± 1.9 | 3.0 ± 1.2 |
| Quercetin-3-rhamnoside | 19.1 ± 2.1 | 108.4 ± 4.6 | 104.6 ± 4.6 | 3.9 ± 1.4 | 3.5 ± 0.9 |
| Rutin (Quercetin-3-rutinoside) | 22.5 ± 1.6 | 99.3 ± 1.1 | 42.9 ± 2.9 | 4.6 ± 2.3 | 4.1 ± 0.8 |
| Kaempferol | 10.6 ± 3.9 | 93.7 ± 3.7 | 1.8 ± 0.3 | 4.5 ± 1.2 | 3.6 ± 1.1 |
| Kaempferol-3-rutinoside | 30.6 ± 3.9 | 108.7 ± 5.6 | 63.8 ± 2.1 | 23.9 ± 1.1 | 19.1 ± 0.5 |
| Isorhamnetin | 63.3 ± 2.3 | 103.1 ± 2.4 | 2.8 ± 0.7 | 8.1 ± 3.1 | 7.5 ± 0.9 |
| Isorhamnetin-3-rutinoside | 85.0 ± 2.1 | 115.3 ± 6.2 | 125.0 ± 3.9 | 25.2 ± 2.9 | 26.2 ± 0.7 |
| Naringenin | 23.3 ± 1.4 | 124.1 ± 3.5 | 95.1 ± 4.5 | 7.5 ± 1.1 | 6.2 ± 1.1 |
| Catechin | 19.0 ± 1.1 | 128.1 ± 5.9 | >167 | 18.5 ± 2.2 | 14.1 ± 0.8 |
| Epicatechin | 15.6 ± 2.3 | 115.6 ± 7.9 | >167 | 12.1 ± 2.3 | 9.7 ± 2.1 |
| Caffeic acid | 90.3 ± 4.3 | 125.9 ± 4.7 | 107.3 ± 3.2 | 18.5 ± 0.9 | 13.9 ± 0.7 |
| Chlorogenic acid | 17.5 ± 3.9 | 106.2 ± 2.3 | 24.2 ± 5.3 | 10.5 ± 2.1 | 9.1 ± 1.1 |
| Neochlorogenic acid | 19.6 ± 1.6 | 115.1 ± 5.8 | 26.2 ± 3.1 | 13.1 ± 1.3 | 15.4 ± 3.2 |
| Metabolites | |||||
| Benzoic acid | 145.3 ± 5.8 | 137.6 ± 6.5 | >167 | 128.9 ± 3.8 | 124.1 ± 5.3 |
| 3-Hydroxyphenylacetic acid | 125.3 ± 4.8 | 133.9 ± 5.8 | >167 | 68.9 ± 3.8 | 44.8 ± 5.3 |
| 3,4-Dihydroxyphenylacetic acid | 115.9 ± 1.4 | 135.5 ± 7.3 | >167 | 78.5 ± 1.6 | 74.9 ± 1.7 |
| 4-Methylcatechol | 40.5 ± 3.6 | 129.0 ± 5.2 | >167 | 98.9 ± 3.5 | 94.8 ± 6.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Effect of Phenolic Compounds from Elderflowers on Glucose- and Fatty Acid Uptake in Human Myotubes and HepG2-Cells. Molecules 2017, 22, 90. https://doi.org/10.3390/molecules22010090
Ho GTT, Kase ET, Wangensteen H, Barsett H. Effect of Phenolic Compounds from Elderflowers on Glucose- and Fatty Acid Uptake in Human Myotubes and HepG2-Cells. Molecules. 2017; 22(1):90. https://doi.org/10.3390/molecules22010090
Chicago/Turabian StyleHo, Giang Thanh Thi, Eili Tranheim Kase, Helle Wangensteen, and Hilde Barsett. 2017. "Effect of Phenolic Compounds from Elderflowers on Glucose- and Fatty Acid Uptake in Human Myotubes and HepG2-Cells" Molecules 22, no. 1: 90. https://doi.org/10.3390/molecules22010090






