In Vitro Assessment of the Effect of Antiepileptic Drugs on Expression and Function of ABC Transporters and Their Interactions with ABCC2
Abstract
:1. Introduction
2. Results
2.1. Effects of AEDs on RNA Expression of ABC Transporters
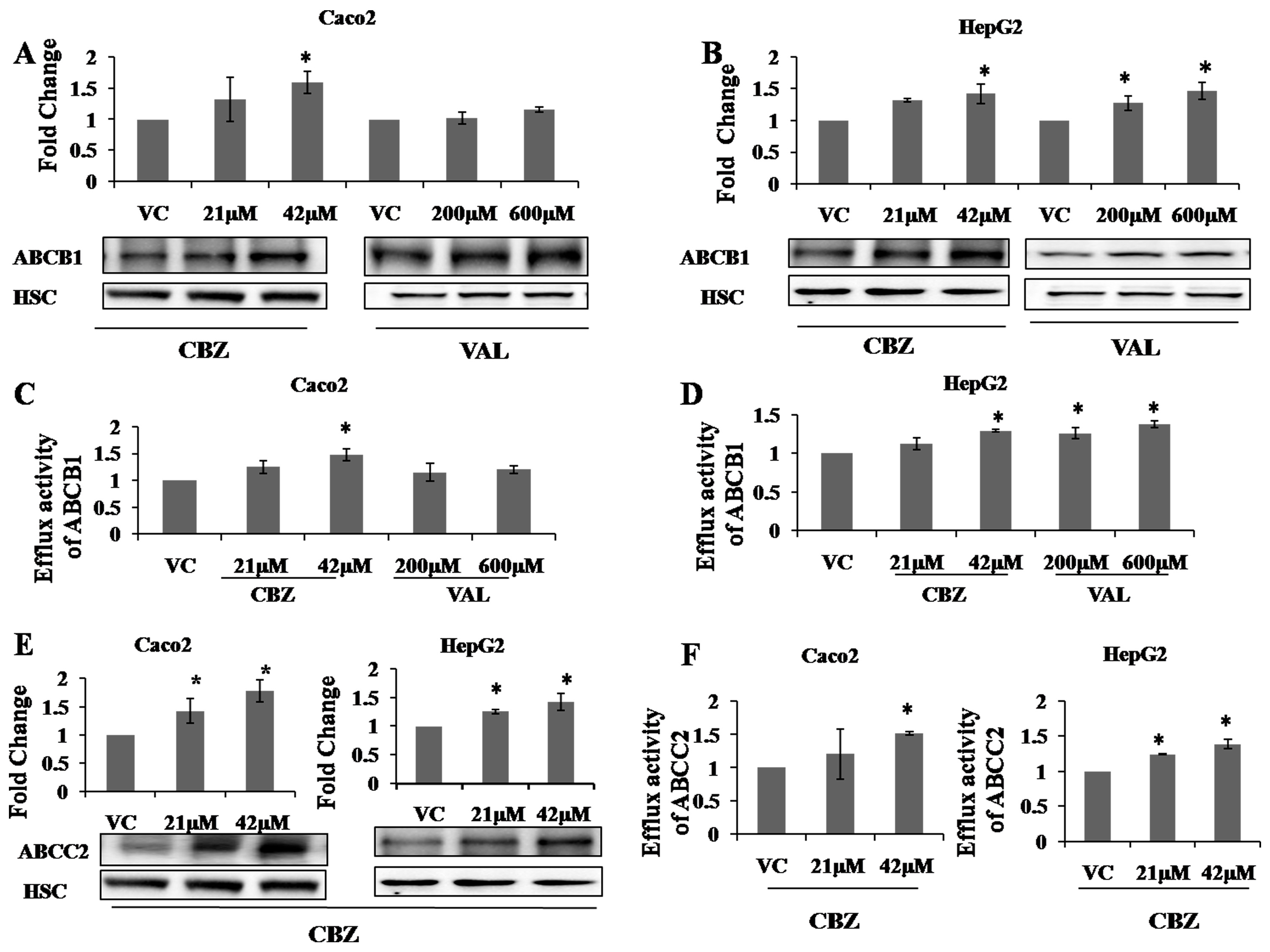
2.2. Effects of AEDs on Protein Expression and Functional Activity of ABC Transporters
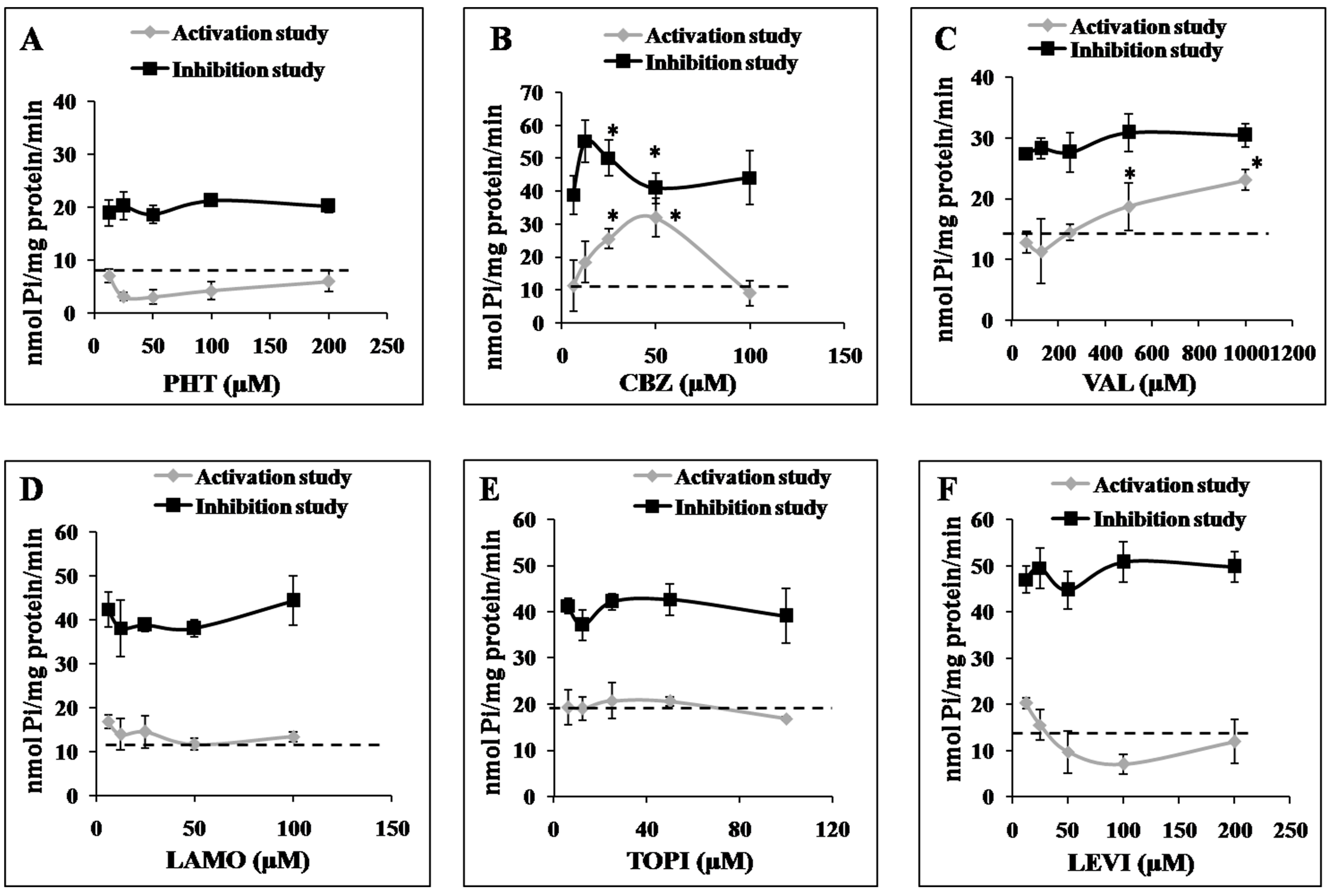
2.3. Effects of AEDs on ABCC2 ATPase Function
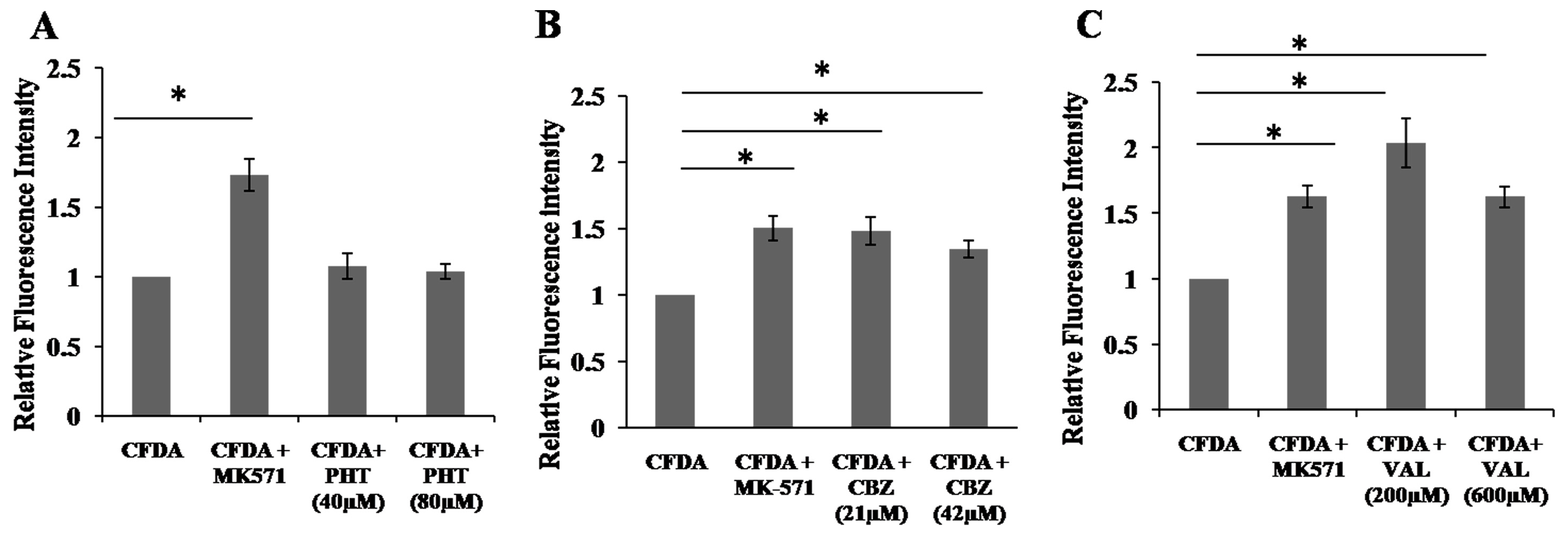
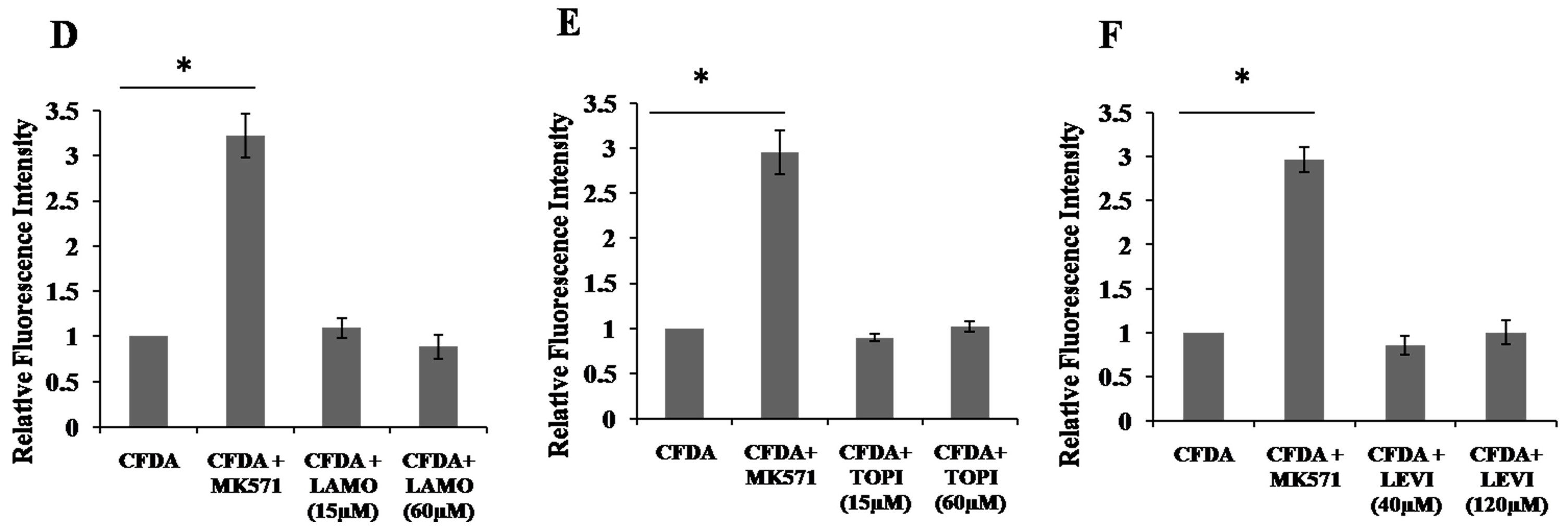
2.4. Inhibition of 5,6-Carboxyfluorescein Efflux (CF) via ABCC2 by AEDs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drug Treatments
4.3. Cell Viability Test
4.4. RNA Extraction and Real Time RT-PCR
4.5. Western Blots
4.6. Functional Activity Assay
4.6.1. Functional Activity Using Rhodamine
4.6.2. Functional Activity Using Carboxyfluorescein Diacetate
4.6.3. Data Analysis
4.7. ATPase Assay
4.8. Efflux Assay Using Fluorescent Substrate-5,6-Carboxyfluorescein Efflux (CF)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wright, J.; Pickard, N.; Whitfield, A.; Hakin, N. A population-based study of the prevalence, clinical characteristics and effect of ethnicity in epilepsy. Seizure 2000, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Tishler, D.M.; Weinberg, K.I.; Hinton, D.R.; Barbaro, N.; Annett, G.M.; Raffel, C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia 1995, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J. Pharmacol. Exp. Ther. 2002, 301, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Marroni, M.; Marchi, N.; Cucullo, L.; Abbott, N.J.; Signorelli, K.; Janigro, D. Vascular and parenchymal mechanisms in multiple drug resistance: A lesson from human epilepsy. Curr. Drug Targets 2003, 4, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, S.M. Mechanisms of antiepileptic drug resistance. Curr. Opin. Neurol. 2003, 16, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Giessmann, T.; May, K.; Modess, C.; Wegner, D.; Hecker, U.; Zschiesche, M.; Dazert, P.; Grube, M.; Schroeder, E.; Warzok, R.; et al. Carbamazepine regulates intestinal P-glycoprotein and multidrug resistance protein MRP2 and influences disposition of talinolol in humans. Clin. Pharmacol. Ther. 2004, 76, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Huai-Yun, H.; Secrest, D.T.; Mark, K.S.; Carney, D.; Brandquist, C.; Elmquist, W.F.; Miller, D.W. Expression of Multidrug Resistance-Associated Protein (MRP) in Brain Microvessel Endothelial Cells. Biochem. Biophys. Res. Commun. 1998, 243, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, A.; Sevlever, G.; Taratuto, A.; Massaro, M.; Rabinowicz, A. Tuberous sclerosis associated with MDR1 gene expression and drug-resistant epilepsy. Pediatr. Neurol. 1999, 21, 731–734. [Google Scholar] [CrossRef]
- Lombardo, L.; Pellitteri, R.; Balazy, M.; Cardile, V. Induction of nuclear receptors and drug resistance in the brain microvascular endothelial cells treated with antiepileptic drugs. Curr. Neurovasc. Res. 2008, 5, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. How to explain multidrug resistance in epilepsy? Epilepsy Curr. 2005, 5, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Oscarson, M.; Zanger, U.M.; Rifki, O.F.; Klein, K.; Eichelbaum, M.; Meyer, U.A. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin. Pharmacol. Ther. 2006, 80, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Caccia, S.; Guiso, G.; Richichi, C.; Gorter, J.A.; Aronica, E.; Aliprandi, M.; Bagnati, R.; Fanelli, R.; D’Incalci, M.; et al. Limbic seizures induce P-glycoprotein in rodent brain: Functional implications for pharmacoresistance. J. Neurosci. 2002, 22, 5833–5839. [Google Scholar] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The Human ATP-Binding Cassette transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.H.; Kim, R.B. Transporters and drug therapy: Implications for drug disposition and disease. Clin. Pharmacol. Ther. 2005, 78, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Elferink, R.O. Mammalian ABC Transporters in Health and Disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P.C. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Shugarts, S.; Benet, L.Z. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 2009, 26, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, A.; Czornyj, L.; Lubienieki, F.; Girardi, E.; Vazquez, S.; D’Giano, C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 2007, 48, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Mao, D.; Liu, L. Research Progress on the Role of ABC Transporters in the Drug Resistance Mechanism of Intractable Epilepsy. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Stieger, B.; Kullak-Ublick, G.A.; Fried, M.; Mueller, S.; Fritschy, J.M.; Wieser, H.G.; Pauli-Magnus, C. Intestinal expression of cytochrome P450 enzymes and ABC transporters and carbamazepine and phenytoin disposition. Acta Neurol. Scand. 2007, 115, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, E.G.; Beck, W.T.; Schuetz, J.D. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol. Pharmacol. 1996, 49, 311–318. [Google Scholar] [PubMed]
- Wen, T.; Liu, Y.C.; Yang, H.W.; Liu, H.Y.; Liu, X.D.; Wang, G.J.; Xie, L. Effect of 21-day exposure of phenobarbital, carbamazepine and phenytoin on P-glycoprotein expression and activity in the rat brain. J. Neurol. Sci. 2008, 270, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.O.; Dahl, M.-L.; Cederberg, J.; Karlsson, M.O.; Sandström, R. Pharmacodynamics of carbamazepine-mediated induction of CYP3A4, CYP1A2, and Pgp as assessed by probe substrates midazolam, caffeine, and digoxin. Clin. Pharmacol. Ther. 2008, 84, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Runge, D.; Köhler, C.; Kostrubsky, V.E.; Jäger, D.; Lehmann, T.; Runge, D.M.; May, U.; Stolz, D.B.; Strom, S.C.; Fleig, W.E.; Michalopoulos, G.K. Induction of cytochrome P450 (CYP)1A1, CYP1A2, and CYP3A4 but not of CYP2C9, CYP2C19, multidrug resistance (MDR-1) and multidrug resistance associated protein (MRP-1) by prototypical inducers in human hepatocytes. Biochem. Biophys. Res. Commun. 2000, 273, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Seegers, U.; Potschka, H.; Löscher, W. Lack of effects of prolonged treatment with phenobarbital or phenytoin on the expression of P-glycoprotein in various rat brain regions. Eur. J. Pharmacol. 2002, 451, 149–155. [Google Scholar] [CrossRef]
- Rubinchik-Stern, M.; Shmuel, M.; Eyal, S. Antiepileptic drugs alter the expression of placental carriers: An in vitro study in a human placental cell line. Epilepsia 2015, 56, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kwan, P.; Zuo, Z.; Baum, L. The transport of antiepileptic drugs by P-glycoprotein. Adv. Drug Deliv. Rev. 2012, 64, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J. Interaction of Antiepileptic Drugs with Human P-Glycoprotein in Vitro. J. Pharmacol. Exp. Ther. 2003, 307, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Lee, J.H.; Yi, J.; Cho, Y.-J.; Heo, K.; Lee, S.H.; Kim, S.W.; Kim, M.-K.; Kim, K.H.; In Lee, B.; et al. A nonsynonymous variation in MRP2/ABCC2 is associated with neurological adverse drug reactions of carbamazepine in patients with epilepsy. Pharmacogenet. Genom. 2010, 20, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Potschka, H.; Fedrowitz, M.; Löscher, W. P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain. Neuroreport 2001, 12, 3557–3560. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J.; Kwan, P.; Butler, E.; de Lange, E.C.M.; van den Berg, D.-J.; Brodie, M.J. P-glycoprotein-mediated efflux of antiepileptic drugs: Preliminary studies in mdr1a knockout mice. Epilepsy Behav. 2002, 3, 427–432. [Google Scholar] [CrossRef]
- Rambeck, B.; Jürgens, U.H.; May, T.W.; Wolfgang Pannek, H.; Behne, F.; Ebner, A.; Gorji, A.; Straub, H.; Speckmann, E.J.; Pohlmann-Eden, B.; et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia 2006, 47, 681–694. [Google Scholar] [CrossRef] [PubMed]
- West, C.L.; Mealey, K.L. Assessment of antiepileptic drugs as substrates for canine P-glycoprotein. Am. J. Vet. Res. 2007, 68, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Luna-Tortós, C.; Römermann, K.; Fedrowitz, M. Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? Problems and approaches in determining which antiepileptic drugs are affected. Curr. Pharm. Des. 2011, 17, 2808–2828. [Google Scholar] [CrossRef] [PubMed]
- Radisch, S.; Dickens, D.; Lang, T.; Bonnett, L.; Arlanov, R.; Johnson, M.R.; Schwab, M.; Marson, A.G.; Pirmohamed, M. A comprehensive functional and clinical analysis of ABCC2 and its impact on treatment response to carbamazepine. Pharmacogenomics J. 2014, 14, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Luna-Tortós, C.; Fedrowitz, M.; Löscher, W. Evaluation of transport of common antiepileptic drugs by human multidrug resistance-associated proteins (MRP1, 2 and 5) that are overexpressed in pharmacoresistant epilepsy. Neuropharmacology 2010, 58, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.; Pirmohamed, M.; Tettey, J.N.; Morgan, P.; Chadwick, D.; Park, B.K. Carbamazepine is not a substrate for P-glycoprotein. Br. J. Clin. Pharmacol. 2002, 51, 345–349. [Google Scholar] [CrossRef]
- Potschka, H.; Fedrowitz, M.; Loscher, W. Brain Access and Anticonvulsant Efficacy of Carbamazepine, Lamotrigine, and Felbamate in ABCC2/MRP2-Deficient TR- Rats. Epilepsia 2003, 44, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Gilibili, R.R.; Chatterjee, S.; Bagul, P.; Mosure, K.W.; Murali, B.V.; Mariappan, T.T.; Mandlekar, S.; Lai, Y. Coproporphyrin-I: A Fluorescent, Endogenous Optimal Probe Substrate for ABCC2 (MRP2) Suitable for Vesicle-Based MRP2 Inhibition Assay. Drug Metab. Dispos. 2017, 45, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Hillgren, K.M.; Keppler, D.; Zur, A.A.; Giacomini, K.M.; Stieger, B.; Cass, C.E.; Zhang, L. Emerging transporters of clinical importance: An update from the International Transporter Consortium. Clin. Pharmacol. Ther. 2013, 94, 52–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamek-Gliszczynski, M.J.; Hoffmaster, K.A.; Tweedie, D.J.; Giacomini, K.M.; Hillgren, K.M. Highlights from the International Transporter Consortium second workshop. Clin. Pharmacol. Ther. 2012, 92, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, S. Therapeutic drug monitoring of antiepileptic drugs. In Handbook of Analytical Separation; Hempe, G., Smith, R.M., Eds.; Elsevier: Amsterdan, The Netherlands, 2004; pp. 221–253. [Google Scholar]
- Haenisch, S.; Laechelt, S.; Bruckmueller, H.; Werk, A.; Noack, A.; Bruhn, O.; Remmler, C.; Cascorbi, I. Down-regulation of ATP-binding cassette C2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Mol. Pharmacol. 2011, 80, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Mohamed, L.A.; Kaddoumi, A. Experimental models for predicting drug absorption and metabolism. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, D.M.; de Vries, E.G.; Noordhoek, L.; van den Berg, E.; van der Pol, M.A.; Müller, M.; Vellenga, E. Activity and expression of the multidrug resistance proteins P-glycoprotein, MRP1, MRP2, MRP3 and MRP5 in de novo and relapsed acute myeloid leukemia. Leukemia 2001, 15, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Cui, Y.; König, J.; Leier, I.; Nies, A. Export pumps for anionic conjugates encoded by MRP genes. Adv. Enzyme Regul. 1999, 39, 237–246. [Google Scholar] [CrossRef]
- Cantz, T.; Nies, A.T.; Brom, M.; Hofmann, A.F.; Keppler, D. MRP2, a human conjugate export pump, is present and transports fluo 3 into apical vacuoles of Hep G2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G522–G531. [Google Scholar] [PubMed]
- van der Kolk, D.M.; De Vries, E.G.; Koning, J.A.; van den Berg, E.; Müller, M.; Vellenga, E. Activity and and and Expression in Acute Hematopoietic of the Myeloid Multidrug Leukemia Resistance Tumor Blood Cell Cells’ Peripheral. Clin. Cancer Res. 1998, 4, 1727–1736. [Google Scholar] [PubMed]
- Aronica, E.; Gorter, J.A.; Ramkema, M.; Redeker, S.; Ozbas-Gerceker, F.; van Vliet, E.A.; Scheffer, G.L.; Scheper, R.J.; van der Valk, P.; Baayen, J.C.; et al. Expression and Cellular Distribution of Multidrug Resistance-related Proteins in the Hippocampus of Patients with Mesial Temporal Lobe Epilepsy. Epilepsia 2004, 45, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Ishihara, H.; Langmann, T.; Schmitz, G.; Stieger, B.; Wieser, H.G.; Yonekawa, Y.; Frei, K. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006, 68, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Seegers, U.; Potschka, H.; Löscher, W. Transient increase of P-glycoprotein expression in endothelium and parenchyma of limbic brain regions in the kainate model of temporal lobe epilepsy. Epilepsy Res. 2002, 51, 257–268. [Google Scholar] [CrossRef]
- Sisodiya, S.M.; Lin, W.R.; Harding, B.N.; Squier, M.V.; Thom, M. Drug resistance in epilepsy: Expression of drug resistance proteins in common causes of refractory epilepsy. Brain 2002, 125, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Gorter, J.A.; Redeker, S.; Van Vliet, E.A.; Ramkema, M.; Scheffer, G.L.; Scheper, R.J.; Van Der Valk, P.; Leenstra, S.; Baayen, J.C.; et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 2005, 46, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Kerb, R.; Weale, M.E.; Brinkmann, U.; Smith, A.; Goldstein, D.B.; Wood, N.W.; Sisodiya, S.M. Association of Multidrug Resistance in Epilepsy with a Polymorphism in the Drug-Transporter Gene ABCB1. N. Engl. J. Med. 2003, 348, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.C.K.; Heron, S.E.; Scheffer, I.E.; Pelekanos, J.T.; McMahon, J.M.; Vears, D.F.; Mulley, J.C.; Berkovic, S.F. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology 2004, 63, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Haerian, B.S.; Lim, K.S.; Tan, C.T.; Raymond, A.A.; Mohamed, Z. Association of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: A systematic review and meta-analysis. Pharmacogenomics 2011, 12, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Bala, K.; Sharma, S.; Gourie-Devi, M.; Baghel, R.; Kaur, H.; Gupta, M.; Talwar, P.; Kukreti, R. Absence of a general association between ABCB1 genetic variants and response to antiepileptic drugs in epilepsy patients. Biochimie 2010, 92, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M.; Mosyagin, I.; Muhle, H.; Jacobsen, T.; Haenisch, S.; Häsler, R.; Faltraco, F.; Remmler, C.; von Spiczak, S.; Kroemer, H.K.; et al. Non-response to antiepileptic pharmacotherapy is associated with the ABCC2 -24C>T polymorphism in young and adult patients with epilepsy. Pharmacogenet. Genom. 2009, 19, 353–362. [Google Scholar] [CrossRef]
- Hilger, E.; Reinthaler, E.M.; Stogmann, E.; Hotzy, C.; Pataraia, E.; Baumgartner, C.; Zimprich, A.; Zimprich, F. Lack of association between ABCC2 gene variants and treatment response in epilepsy. Pharmacogenomics 2012, 13, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Markova, S.; Liu, W.; Gow, J.M.; Baldwin, R.M.; Habashian, M.; Relling, M.V.; Ratain, M.J.; Kroetz, D.L. Functional characterization of ABCC2 promoter polymorphisms and allele-specific expression. Pharmacogenomics J. 2013, 13, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.; Goldring, C.; Morgan, P.; Park, B.K.; Pirmohamed, M. Induction of P-glycoprotein in lymphocytes by carbamazepine and rifampicin: The role of nuclear hormone response elements. Br. J. Clin. Pharmacol. 2006, 62, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Eyal, S.; Lamb, J.G.; Smith-Yockman, M.; Yagen, B.; Fibach, E.; Altschuler, Y.; White, H.S.; Bialer, M. The antiepileptic and anticancer agent, valproic acid, induces P-glycoprotein in human tumour cell lines and in rat liver. Br. J. Pharmacol. 2006, 149, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, L.; Svecova, L.; Anzenbacherova, E.; Vrzal, R.; Staud, F.; Dvorak, Z.; Ulrichova, J.; Anzenbacher, P.; Pavek, P. Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metab. Dispos. 2007, 35, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Alms, D.; Fedrowitz, M.; Römermann, K.; Noack, A.; Löscher, W. Marked differences in the effect of antiepileptic and cytostatic drugs on the functionality of P-glycoprotein in human and rat brain capillary endothelial cell lines. Pharm. Res. 2014, 31, 1588–1604. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into regulation of protein abundance from proteomics and transcriptomis analyses. Nat. Rev. Genet. 2013, 13, 227–232. [Google Scholar]
- Grewal, G.K.; Singh, K.D.; Kanojia, N.; Rawat, C.; Kukal, S.; Jajodia, A.; Singhal, A.; Misra, R.; Nagamani, S.; Muthusamy, K.; et al. Exploring the Carbamazepine Interaction with Human Pregnane X Receptor and Effect on ABCC2 Using in Vitro and in Silico Approach. Pharm. Res. 2017, 34, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Stȩpień, K.M.; Tomaszewski, M.; Tomaszewska, J.; Czuczwar, S.J. The multidrug transporter P-glycoprotein in pharmacoresistance to antiepileptic drugs. Pharmacol. Rep. 2012, 64, 1011–1019. [Google Scholar] [CrossRef]
- Munić, V.; Kelnerić, Ž.; Mikac, L.; Eraković Haber, V. Differences in assessment of macrolide interaction with human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase activity and cellular accumulation assays. Eur. J. Pharm. Sci. 2010, 41, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.P.; Adeyeye, M.C.; Yang, Z.; Shen, D.D. Valproic acid uptake by bovine brain microvessel endothelial cells: Role of active efflux transport. Epilepsy Res. 2004, 58, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, C.J.; Miller, D.W. A fluorometric screening assay for drug efflux transporter activity in the blood-brain barrier. Pharm. Res. 2005, 22, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Baltes, S.; Fedrowitz, M.; Luna Tortos, C.; Potschka, H.; Loscher, W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J. Pharmacol. Exp.Ther. 2007, 320, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lü, Y.; Huang, L. Detecting overexpression of P-glycoprotein-multidrug resistance gene in epileptic patient’s peripheral blood lymphocytes. Chin. J. Neurol. 2002, 35, 348–350. [Google Scholar]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-Resistant Epilepsy: Multiple Hypotheses, Few Answers. Front. Neurol. 2017, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Chu, K.; Shin, S.A.; Jung, K.-H.; Lee, S.-T.; Lee, Y.-S.; Moon, J.; Lee, D.Y.; Lee, J.S.; Lee, D.S.; Lee, S.K. Clinical Applications of Simultaneous PET/MR Imaging Using (R)-[11C]-Verapamil with Cyclosporin A: Preliminary Results on a Surrogate Marker of Drug-Resistant Epilepsy. AJNR Am. J. Neuroradiol. 2016, 37, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Karch, R.; Zeitlinger, M.; Liu, J.; Koepp, M.J.; Asselin, M.-C.; Sisodiya, S.M.; Hainfellner, J.A.; Wadsak, W.; Mitterhauser, M.; et al. In vivo P-glycoprotein function before and after epilepsy surgery. Neurology 2014, 83, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Koepp, M. ABC Transporters and Drug Resistance in Patients with Epilepsy. Curr. Pharm. Des. 2016, 22, 5793–5807. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, A.; Dugois, P.; Tandeo, D.; Peltekian, M.; Kong Thoo Lin, P. Synthesis, cytotoxicity and DNA binding of oxoazabenzo[de]anthracenes derivatives in colon cancer Caco-2 cells. Eur. J. Med. Chem. 2013, 69, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.V.; Pande, P.; Patton, W.F. Sensitive and specific fluorescent probes for functional analysis of the three major types of mammalian ABC transporters. PLoS ONE 2011, 6, e22429. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| DRUG | DRUG Concentration | Caco2 | HepG2 | Caco2 | HepG2 | Caco2 | HepG2 | Caco2 | HepG2 | Caco2 | HepG2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCB1 | ABCC1 | ABCC2 | ABCG2 | CYP3A4 | |||||||
| Phenytoin (PHT) | 40 µM | 1.09 ± 0.22 | 1.23 ± 0.15 * | 1.17 ± 0.36 | 1.02 ± 0.12 | 1.09 ± 0.28 | 0.91 ± 0.25 | 1.19 ± 0.28 | 0.98 ± 0.31 | 1.25 ± 0.01 * | 1.34 ± 0.01 * |
| 80 µM | 1.02 ± 0.21 | 1.39 ± 0.31 * | 1.01 ± 0.39 | 0.69 ± 0.01 | 1.04 ± 0.34 | 1.00 ± 0.19 | 1.17 ± 0.22 | 1.08 ± 0.14 | 1.28 ± 0.01 * | 2.21 ± 0.26 * | |
| Carbamazepine (CBZ) | 21 µM | 2.35 ± 0.07 * | 1.69 ± 0.46 * | 1.44 ± 0.37 * | 1.12 ± 0.33 | 1.64 ± 0.15 * | 1.68 ± 0.16 * | 0.84 ± 0.23 | 0.95 ± 0.28 | 2.55 ± 0.08 * | 1.49 ± 0.09 * |
| 42 µM | 2.27 ± 0.01 * | 2.26 ± 0.33 * | 1.19 ± 0.12 | 1.57 ± 0.17 * | 1.73 ± 0.23 * | 1.74 ± 0.12 * | 0.81 ± 0.16 | 1.37 ± 0.09 * | 3.30 ± 0.15 * | 2.83 ± 0.25 * | |
| Valproate (VAL) | 200 µM | 0.64 ± 0.22 * | 2.23 ± 0.21 * | 1.15 ± 0.22 | 1.10 ± 0.27 | 0.62 ± 0.24 | 0.55 ± 0.19 | 1.13 ± 0.35 | 1.05 ± 0.08 | 2.11 ± 0.04 * | 2.68 ± 0.13 * |
| 600 µM | 0.55 ± 0.14 * | 3.04 ± 0.27 * | 1.15 ± 0.20 | 1.33 ± 0.64 | 0.83 ± 0.04 | 0.66 ± 0.11 | 1.07 ± 0.37 | 1.41 ± 0.26 * | 3.16 ± 0.16 * | 3.52 ± 0.27 * | |
| Lamotrigine (LAMO) | 15 µM | 1.24 ± 0.13 | 1.06 ± 0.01 | 1.17 ± 0.32 | 1.04 ± 0.33 | 0.87 ± 0.24 | 0.88 ± 0.14 | 1.31 ± 0.06 | 0.80 ± 0.08 | 1.09 ± 0.01 | 1.08 ± 0.09 |
| 60 µM | 1.12 ± 0.09 | 1.26 ± 0.15 | 0.84 ± 0.20 | 1.01 ± 0.04 | 1.07 ± 0.26 | 1.25 ± 0.16 | 0.89 ± 0.02 | 1.00 ± 0.01 | 0.90 ± 0.20 | 1.06 ± 0.27 | |
| Topiramate (TOPI) | 15 µM | 1.51 ± 0.16 * | 1.05 ± 0.14 | 0.97 ± 0.11 | 0.97 ± 0.09 | 1.02 ± 0.13 | 0.94 ± 0.07 | 1.40 ± 0.13 | 1.24 ± 0.23 | 1.23 ± 0.01 | 1.69 ± 0.33 |
| 60 µM | 1.28 ± 0.06 | 0.89 ± 0.14 | 1.06 ± 0.01 | 1.45 ± 0.09 * | 1.27 ± 0.26 | 1.55 ± 0.08 * | 1.59 ± 0.17 * | 1.41 ± 0.03 | 1.64 ± 0.15 * | 2.14 ± 0.24 * | |
| Levetiracetam (LEVI) | 40 µM | 0.90 ± 0.13 | 0.75 ± 0.11 | 0.90 ± 0.10 | 1.02 ± 0.28 | 0.87 ± 0.11 | 0.78 ± 0.11 | 1.00 ± 0.05 | 1.06 ± 0.15 | 1.13 ± 0.19 | 0.96 ± 0.15 |
| 120 µM | 1.10 ± 0.16 | 0.95 ± 0.20 | 1.07 ± 0.14 | 1.07 ± 0.33 | 1.24 ± 0.01 | 0.72 ± 0.09 | 0.93 ± 0.04 | 0.87 ± 0.08 | 1.08 ± 0.21 | 1.13 ± 0.04 | |
| Primers | Sequence | |
|---|---|---|
| ABCB1 | Forward | GCCTGGCAGCTGGAAGACAAATAC |
| Reverse | ATGGCCAAAATCACAAGGGTTAGC | |
| ABCC1 | Forward | TGTGTGGGCAACTGCATCG |
| Reverse | GTTGGTTTCCATTTCAGATGACATCCG | |
| ABCC2 | Forward | ATATAAGAAGGCATTGACCC |
| Reverse | ATCTGTAGAACACTTGACC | |
| ABCG2 | Forward | GAAGAGTGGCTTTCTACCTT |
| Reverse | GTCCCAGGATGGCGTTGA | |
| CYP3A4 | Forward | TTGGAAGTGGACCCAGAAAC |
| Reverse | CTGGTGTTCTCAGGCACAGA | |
| B2M | Forward | GGCATTCCTGAAGCTGACAG |
| Reverse | TGGATGACGTGAGTAAACCTG | |
| GAPDH | Forward | ACATCGCTCAGACACCATG |
| Reverse | TGTAGTTGAGGTCAATGAAGGG | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grewal, G.K.; Kukal, S.; Kanojia, N.; Madan, K.; Saso, L.; Kukreti, R. In Vitro Assessment of the Effect of Antiepileptic Drugs on Expression and Function of ABC Transporters and Their Interactions with ABCC2. Molecules 2017, 22, 1484. https://doi.org/10.3390/molecules22101484
Grewal GK, Kukal S, Kanojia N, Madan K, Saso L, Kukreti R. In Vitro Assessment of the Effect of Antiepileptic Drugs on Expression and Function of ABC Transporters and Their Interactions with ABCC2. Molecules. 2017; 22(10):1484. https://doi.org/10.3390/molecules22101484
Chicago/Turabian StyleGrewal, Gurpreet Kaur, Samiksha Kukal, Neha Kanojia, Krateeka Madan, Luciano Saso, and Ritushree Kukreti. 2017. "In Vitro Assessment of the Effect of Antiepileptic Drugs on Expression and Function of ABC Transporters and Their Interactions with ABCC2" Molecules 22, no. 10: 1484. https://doi.org/10.3390/molecules22101484






